Water Extract of Fritillariae thunbergii Bulbus Inhibits RANKL-Mediated Osteoclastogenesis and Ovariectomy-Induced Trabecular Bone Loss
Abstract
:1. Introduction
2. Results and Discussion
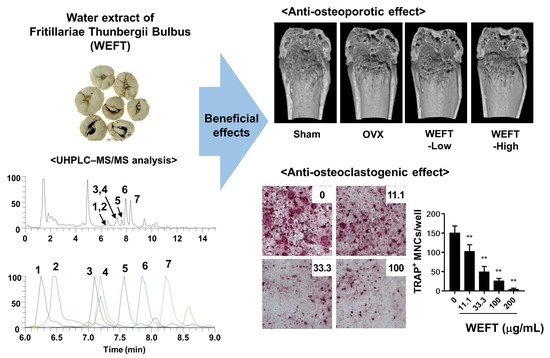
2.1. WEFT Inhibits RANKL-Induced Osteoclast Differentiation
2.2. WEFT Inhibits RANKL-Induced NFATc1 Expression
2.3. WEFT Attenuates OVX-Induced Bone Loss in Mice
2.4. Phytochemical Profiles of WEFT
3. Materials and Methods
3.1. Materials
3.2. BMM Preparation
3.3. Cell Culture
3.4. Cell Viability
3.5. Osteoclast Differentiation
3.6. TRAP Activity and Staining
3.7. Western Blot Analysis
3.8. qPCR Analysis
3.9. In Vivo Study
3.10. Trabecular Bone Analysis
3.11. Histological Analysis
3.12. UHPLC-MS/MS Analysis
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Seeman, E. Reduced bone formation and increased bone resorption: Rational targets for the treatment of osteoporosis. Osteoporos. Int. 2003, 14 (Suppl. 3), 2–8. [Google Scholar] [CrossRef] [PubMed]
- Datta, H.K.; Ng, W.F.; Walker, J.A.; Tuck, S.P.; Varanasi, S.S. The cell biology of bone metabolism. J. Clin. Pathol. 2008, 61, 577–587. [Google Scholar] [CrossRef]
- Eriksen, E.F. Cellular mechanisms of bone remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Unnanuntana, A.; Gladnick, B.P.; Donnelly, E.; Lane, J.M. The assessment of fracture risk. J. Bone Jt. Surg. Am. 2010, 92, 743–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Kobayashi, N.; Kadono, Y.; Naito, A.; Matsumoto, K.; Yamamoto, T.; Tanaka, S.; Inoue, J. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J. 2001, 20, 1271–1280. [Google Scholar] [CrossRef] [Green Version]
- Darnay, B.G.; Ni, J.; Moore, P.A.; Aggarwal, B.B. Activation of NF-kappaB by RANK requires tumor necrosis factor receptor-associated factor (TRAF) 6 and NF-kappaB-inducing kinase. Identification of a novel TRAF6 interaction motif. J. Biol. Chem. 1999, 274, 7724–7731. [Google Scholar] [CrossRef] [Green Version]
- Teitelbaum, S.L.; Ross, F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003, 4, 638–649. [Google Scholar] [CrossRef]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Kim, J.H.; Lee, J.; Jin, H.M.; Kook, H.; Kim, K.K.; Lee, S.Y.; Kim, N. MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood 2007, 109, 3253–3259. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Takami, M.; Yamada, A.; Wang, X.; Koga, T.; Hu, X.; Tamura, T.; Ozato, K.; Choi, Y.; Ivashkiv, L.B.; et al. Interferon regulatory factor-8 regulates bone metabolism by suppressing osteoclastogenesis. Nat. Med. 2009, 15, 1066–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Hung, A.; Li, M.; Yang, A.W.H. Fritillariae Thunbergii Bulbus: Traditional Uses, Phytochemistry, Pharmacodynamics, Pharmacokinetics and Toxicity. Int. J. Mol. Sci. 2019, 20, 1667. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.L.Y.; Li, Z.; Tang, L.; Wu, S.; Yan, X.; Peng, C. Comparative Studies on Antitussive Effect between Fritillaria unibracteata Hisao et K.C.Hisa and Fritillaria thunbergii Miq with Chemical Stimulation Induced Cough Method. Lishizhen Med. Mater. Med. Res. 2012, 23, 2522–2525. [Google Scholar]
- Wu, X.; Chan, S.W.; Ma, J.; Li, P.; Shaw, P.C.; Lin, G. Investigation of association of chemical profiles with the tracheobronchial relaxant activity of Chinese medicinal herb Beimu derived from various Fritillaria species. J. Ethnopharmacol. 2018, 210, 39–46. [Google Scholar] [CrossRef]
- Zhou, Y.; Ji, H.; Lin, B.Q.; Jiang, Y.; Li, P. The effects of five alkaloids from Bulbus Fritillariae on the concentration of cAMP in HEK cells transfected with muscarinic M(2) receptor plasmid. Am. J. Chin. Med. 2006, 34, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Yoon, Y.P.; Woo, K.W.; Kim, J.H.; Min, S.Y.; Lee, H.J.; Lee, S.K.; Hong, J.H.; Lee, K.R.; Lee, C.J. Verticine, ebeiedine and suchengbeisine isolated from the bulbs of Fritillaria thunbergii Miq. inhibited the gene expression and production of MUC5AC mucin from human airway epithelial cells. Phytomedicine 2016, 23, 95–104. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Zhang, P.; Yang, B.; Wu, H.; Guo, X.; Li, Y.; Zhang, Y. Sensitive UHPLC-MS/MS quantitation and pharmacokinetic comparisons of multiple alkaloids from Fuzi- Beimu and single herb aqueous extracts following oral delivery in rats. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1058, 24–31. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Zhu, W.; Zhang, H.; Yan, Z.; Liu, H. Comparative pharmacokinetic studies of peimine and peiminine in rat plasma by LC-MS-MS after oral administration of Fritillaria thunbergii Miq. and Fritillaria thunbergii Miq.—Glycyrrhiza uralensis Fisch. couple extract. Pharmazie 2011, 66, 684–689. [Google Scholar] [PubMed]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-Hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F.; et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Y.K.; Harris, S.; Ahuja, S.S.; Bonewald, L.F. MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J. Bone Miner. Res. 2002, 17, 2068–2079. [Google Scholar] [CrossRef]
- Kurata, K.; Heino, T.J.; Higaki, H.; Vaananen, H.K. Bone marrow cell differentiation induced by mechanically damaged osteocytes in 3D gel-embedded culture. J. Bone Miner. Res. 2006, 21, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Asagiri, M.; Sato, K.; Usami, T.; Ochi, S.; Nishina, H.; Yoshida, H.; Morita, I.; Wagner, E.F.; Mak, T.W.; Serfling, E.; et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 2005, 202, 1261–1269. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Lee, S.H.; Ha Kim, J.; Choi, Y.; Kim, N. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP). Mol. Endocrinol. 2008, 22, 176–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yagi, M.; Miyamoto, T.; Sawatani, Y.; Iwamoto, K.; Hosogane, N.; Fujita, N.; Morita, K.; Ninomiya, K.; Suzuki, T.; Miyamoto, K.; et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 2005, 202, 345–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.; Bi, Y.N.; Zhang, P.Y.; Yuan, X.M.; Liu, Y.; Zhang, Y.; Huang, J.Y.; Zhou, K. Optimization of the Time Window of Interest in Ovariectomized Imprinting Control Region Mice for Antiosteoporosis Research. Biomed. Res. Int. 2017, 2017, 8417814. [Google Scholar] [CrossRef] [Green Version]
- Wronski, T.J.; Cintron, M.; Doherty, A.L.; Dann, L.M. Estrogen treatment prevents osteopenia and depresses bone turnover in ovariectomized rats. Endocrinology 1988, 123, 681–686. [Google Scholar] [CrossRef]
- Streicher, C.; Heyny, A.; Andrukhova, O.; Haigl, B.; Slavic, S.; Schuler, C.; Kollmann, K.; Kantner, I.; Sexl, V.; Kleiter, M.; et al. Estrogen Regulates Bone Turnover by Targeting RANKL Expression in Bone Lining Cells. Sci. Rep. 2017, 7, 6460. [Google Scholar] [CrossRef] [Green Version]
- Krum, S.A.; Miranda-Carboni, G.A.; Hauschka, P.V.; Carroll, J.S.; Lane, T.F.; Freedman, L.P.; Brown, M. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J. 2008, 27, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Riggs, B.L.; Khosla, S.; Melton, L.J., 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 2002, 23, 279–302. [Google Scholar] [CrossRef]
- Syed, F.A.; Oursler, M.J.; Hefferanm, T.E.; Peterson, J.M.; Riggs, B.L.; Khosla, S. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos. Int. 2008, 19, 1323–1330. [Google Scholar] [CrossRef] [Green Version]
- Ambrosi, T.H.; Scialdone, A.; Graja, A.; Gohlke, S.; Jank, A.M.; Bocian, C.; Woelk, L.; Fan, H.; Logan, D.W.; Schurmann, A.; et al. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell 2017, 20, 771–784 e776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muruganandan, S.; Govindarajan, R.; Sinal, C.J. Bone Marrow Adipose Tissue and Skeletal Health. Curr. Osteoporos Rep. 2018, 16, 434–442. [Google Scholar] [CrossRef]
- Syed, M.; Khan, M.N.; Khadim, A.; Shadab, H.; Perveen, A.; El-Seedi, H.R.; Musharraf, S.G. Chemical fingerprinting of three Anemone species and an adulteration study to detect cross mixing of medicinal plants by HPLC-HR-ESI-MS/MS method. J. King Saud Univ. Sci. 2021, 33, 101461. [Google Scholar] [CrossRef]
- Suh, W.S.; Lee, S.Y.; Park, J.E.; Kim, D.H.; Kim, S.Y.; Lee, K.R. Two new steroidal alkaloids from the bulbs of Fritillaria Thunbergii. Heterocycles 2018, 96, 925–934. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.L.; Xin, G.Z.; Shi, Z.Q.; Ren, M.T.; Qi, L.W.; Li, H.J.; Li, P. Characterization and identification of steroidal alkaloids in Fritillaria species using liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 7109–7122. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Jang, S.A.; Kim, T.; Ha, H. Forsythia suspensa Protects against Bone Loss in Ovariectomized Mice. Nutrients 2019, 11, 1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- State Pharmacopoeia Commission of the PRC. Pharmacopoeia of The People’s Republic of China: Volume I; People’s Medical Publishing House: Beijing, China, 2015; Volume 1. [Google Scholar]
- Liu, S.; Yang, T.; Ming, T.W.; Gaun, T.K.W.; Zhou, T.; Wang, S.; Ye, B. Isosteroid alkaloids with different chemical structures from Fritillariae cirrhosae bulbus alleviate LPS-induced inflammatory response in RAW 264.7 cells by MAPK signaling pathway. Int. Immunopharmacol. 2020, 78, 106047. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Lv, Z.T.; Zhou, C.H.; Liang, S.; Huang, W.; Wang, Z.G.; Zhu, W.T.; Wang, Y.T.; Jing, X.Z.; Lin, H.; et al. Peimine suppresses interleukin1betainduced inflammation via MAPK downregulation in chondrocytes. Int. J. Mol. Med. 2019, 43, 2241–2251. [Google Scholar] [CrossRef]
- Jimi, E.; Takakura, N.; Hiura, F.; Nakamura, I.; Hirata-Tsuchiya, S. The Role of NF-kappaB in Physiological Bone Development and Inflammatory Bone Diseases: Is NF-kappaB Inhibition “Killing Two Birds with One Stone”? Cells 2019, 8, 1636. [Google Scholar] [CrossRef] [Green Version]
- Hotokezaka, H.; Sakai, E.; Ohara, N.; Hotokezaka, Y.; Gonzales, C.; Matsuo, K.; Fujimura, Y.; Yoshida, N.; Nakayama, K. Molecular analysis of RANKL-independent cell fusion of osteoclast-like cells induced by TNF-alpha, lipopolysaccharide, or peptidoglycan. J. Cell. Biochem. 2007, 101, 122–134. [Google Scholar] [CrossRef]
- Zhu, M.; Xu, W.; Jiang, J.; Wang, Y.; Guo, Y.; Yang, R.; Chang, Y.; Zhao, B.; Wang, Z.; Zhang, J.; et al. Peiminine Suppresses RANKL-Induced Osteoclastogenesis by Inhibiting the NFATc1, ERK, and NF-κB Signaling Pathways. Front. Endocrinol. 2021, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; An, H.; Shim, K.S.; Kim, T.; Lee, K.J.; Hwang, Y.H.; Ma, J.Y. Ethanol extract of Atractylodes macrocephala protects bone loss by inhibiting osteoclast differentiation. Molecules 2013, 18, 7376–7388. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| No | Rt (Min) | Calculated (m/z) | Estimated (m/z) | Adducts | Error (ppm) | Elemental Composition | MS/MS Fragments (m/z) | Identifications |
|---|---|---|---|---|---|---|---|---|
| 1 | 6.25 | 590.3687 | 590.3686 | [M + H]+ | −0.259 | C33H51NO8 | 590.3686, 572.3564 | Peimisine glucoside [35] |
| 2 | 6.49 | 444.3108 | 444.3105 | [M + H]+ | −0.718 | C27H41NO4 | 444.3105, 426.301, 114.0915 | Yibeissine * |
| 3 | 7.10 | 594.4000 | 594.3998 | [M + H]+ | −0.444 | C33H55NO8 | 594.3998, 576.3887 | Peiminoside [35] |
| 4 | 7.19 | 592.3844 | 592.3840 | [M + H]+ | −0.609 | C33H53NO8 | 592.384, 574.373 | Sipeimine Glucoside * |
| 5 | 7.57 | 428.3159 | 428.3157 | [M + H]+ | −0.504 | C27H41NO3 | 428.3156, 410.3038 | Peimisine * |
| 6 | 7.85 | 432.3472 | 432.3469 | [M + H]+ | −0.757 | C27H45NO3 | 432.3469, 414.3364 | Peimine [35] |
| 7 | 8.22 | 430.3316 | 430.3314 | [M + H]+ | −0.488 | C27H43NO3 | 430.3311, 412.3201 | Peiminine * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, K.-S.; Gu, D.-R.; Hwang, Y.-H.; Yang, H.; Ryuk, J.-A.; Ha, H. Water Extract of Fritillariae thunbergii Bulbus Inhibits RANKL-Mediated Osteoclastogenesis and Ovariectomy-Induced Trabecular Bone Loss. Molecules 2022, 27, 169. https://doi.org/10.3390/molecules27010169
Shim K-S, Gu D-R, Hwang Y-H, Yang H, Ryuk J-A, Ha H. Water Extract of Fritillariae thunbergii Bulbus Inhibits RANKL-Mediated Osteoclastogenesis and Ovariectomy-Induced Trabecular Bone Loss. Molecules. 2022; 27(1):169. https://doi.org/10.3390/molecules27010169
Chicago/Turabian StyleShim, Ki-Shuk, Dong-Ryun Gu, Youn-Hwan Hwang, Hyun Yang, Jin-Ah Ryuk, and Hyunil Ha. 2022. "Water Extract of Fritillariae thunbergii Bulbus Inhibits RANKL-Mediated Osteoclastogenesis and Ovariectomy-Induced Trabecular Bone Loss" Molecules 27, no. 1: 169. https://doi.org/10.3390/molecules27010169