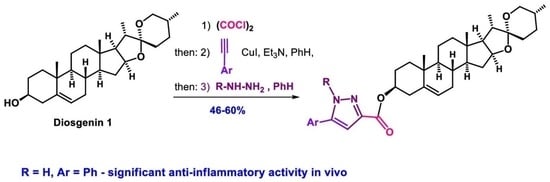
One-Pot Synthesis of Steroidal Ynediones (4–8)
A solution of diosgenin (800 mg, 1.93 mmol) in CHCl3 (10 mL) was added dropwise to a cold stirred solution of oxalyl chloride (980 mg (0.66 mL), 7.72 mmol) in CHCl3 (5 mL) in argon atmosphere at 0 °C for 1 h and the reaction mixture was stirred at room temperature for 3 h. The solvent was removed under reduced pressure, the residue was treated with CHCl3 (3mL) and additionally evaporated. This procedure was repeated three times for removing the trace of oxalyl chloride and diluted with C6H6 (10 mL) in an argon flow. The corresponding aryl acetylene 3a–e (1.00 mmol), CuI (19 mg, 0.1 mmol) and Et3N (0.138 mL, 1.00 mmol) were added subsequently in an argon flow at room temperature. The mixture was heated under stirring at 40 °C for 12 h (TLC), after that solvent was removed under reduced pressure, and the residue was purified by column chromatography (eluent petroleum ether–ether, 20:1) to give compounds (4–8).
Compound 4. (4S,5′R,6aR,6bS,8aS,8bR,9S,10R,11aS,12aS,12bS)-5′,6a,8a,9-Tetramet- hyl-1,3,3′,4,4′,5,5′,6,6a,6b,6′,7,8,8a,8b,9,11a,12,12a,12b-icosahydrospiro[naphtho [2′,1′:4,5]- indeno [2,1-b]furan-10,2′-pyran]-4-yl 2-oxo-4-phenylbut-3-ynoate [(22R,25R)-spirost-5-en -3β-yl 2-oxo-4-phenylbut-3-ynoate] (4). Yield 48%. Yellowish needles. M.p. 209–212 °C (decomp.) (petroleum ether–diethyl ether, 20:1). [α]D27—88.5 (c 0.4, CHCl3). 1H NMR (600 MHz, CDCl3, δ, ppm): 0.74 (3H, d, J = 6.8 Hz, H-27), 0.77 (3H, s, H-19), 0.98 (3H, d, J = 7.0 Hz, H-21), 1.01 (1H, m, H-9), 1.08 (3H, s, H-18), 1.10–1.22 (3H, m, H-1,12,14), 1.25–1.33 м (1H, m, H-15), 1.44–1.50 (2H, m, H-11,24), 1.54 (1H, m, H-11), 1.58–1.69 (6H, m, H-7,8,23,23,24, 25), 1.75 (1H, dm, J = 8.9 Hz, H-12), 1.79 (2H, m, H-2,17), 1.88 (1H, m, H-20), 1.93 (1H, dt, J = 13.5, 3.3 Hz, H-1), 1.98–2.06 (3H, m, H-2,7,15), 2.46 (1H, ddd, J = 13.3, 4.9, 1.9 Hz, H-4), 2.52 (1H, td, J = 11.3, 2.0 Hz, H-4), 3.38 (1H, t, J = 11.0 Hz, H-26), 3.48 (1H, dd, J = 11.0, 2.3 Hz, H-26), 4.42 (1H, dd, J = 15.2, 7.4 Hz, H-16), 4.82 (1H, m, H-3), 5.43 (1H, dd, J = 3.5, 1.9 Hz, H-6). 7.43 (2H, dd, J = 8.2, 7.8 Hz, H-3″,5″), 7.53 (1H, t, J = 7.8 Hz, H-4″), 7.67 (2H, d, J = 8.2 Hz, H-2″,6″). 13C NMR (151 MHz, CDCl3, δ, ppm): 14.5 (C-21), 16.2 (C-18), 17.1 (C-27), 19.3 (C-19), 20.8 (C-11), 27.4 (C-2), 28.8 (C-24), 30.3 (C-25), 31.4 (C-8), 31.4 (C-23), 31.8 (C-15), 32.1 (C-7), 36.7 (C-10), 36.9 (C-1), 37.7 (C-4), 39.7 (C-12), 40.3 (C-13), 41.6 (C-20), 49.9 (C-9), 56.4 (C-14), 62.2 (C-17), 66.8 (C-26), 77.4 (C-3), 80.8 (C-16), 87.2 (C-3′), 97.8 (C-4′), 109.3 (C-22), 119.2 (C-1″), 123.1 (C-6), 128.8 (C-3″,5″), 131.7 (C-4″), 133.8 (C-2″,6″), 139.0 (C-5), 158.8 (C-1′), 169.9 (C-2′). IR (KBr, ν, cm-1): 2195 (C≡C), 1740, 1680(C=O), 1066, 1051, 1024, 1007 (C-O-C), 1597, 814, 760, 688 (C=C). HR-MS, m/z (Irel., %): 570 (0.2), 397 (21), 396 (42), 321 (20), 139 (34), 129 (100), 105 (22), 97 (18), 93 (18), 69 (23), 55.0 (32), 41 (20). Calcd. C37H46O5, m/z [M]+ 570.3340. Found, m/z: 570.3339. X-ray structural analysis of compound 4: C37H46O5, M 570.74, monoclinic, P21, a 11.4807(5), b 7.2087(2), c 19.2482(7) Å, β 93.359(2)° V 1590.3(1) Å3, Z 2, Dcalcd 1.192 g·cm−3, μ(Mo-Kα) 0.078 mm−1, F(000) 616, (θ 1.66–25.05°, completeness 99.9%), colorless, (1.00 × 0.77 × 0.15) mm3, transmission 0.8010–0.8620. The intensities of 5632 independent reflections were measured (Rint 0.0401), 383 parameters, R1 0.0473 (for 4473 observed I > 2σ(I)). The final refinement parameters: wR2 0.1360, GOOF 1.065, largest diff. peak and hole 0.469 and −0.240 e.A−3. Crystallographic data for structure 4 have been deposited at the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC 2077781.
Compound 5. (4S,5′R,6aR,6bS,8aS,8bR,9S,10R,11aS,12aS,12bS)-5′,6a,8a,9-Tetramet- hyl-1,3,3′,4,4′,5,5′,6,6a,6b,6′,7,8,8a,8b,9,11a,12,12a,12b-icosahydrospiro[naphtho[2′,1′:4,5]indeno[2,1-b]furan-10,2′-pyran]-4-yl 4-(4-ethylphenyl)-2-oxobut-3-ynoate [(22R,25R)-spirost-5-en-3β-yl 4-(4-ethylphenyl)-2-oxobut-3-ynoate] (5). Yield 35%. Yellowish solid. M.p. 79 °C (decomp.). [α]D25—72.6 (c 0.2, CHCl3). 1H NMR (400 MHz, CDCl3, δ, ppm): 0.74 (3H, d, J = 6.8 Hz, H-27), 0.77 (3H, s, H-19), 0.98 (3H, d, J = 7.0 Hz, H-21), 1.01 (1H, m, H-9), 1.08 (3H, s, H-18), 1.10–1.34 (7H, m, H-1,12,14,15, C8″H3), 1.40–2.05 (17H, m, H-1,2,2,7,7,8,11,11,12,15,17,20,23,23,24,24,25), 2.49 (2H, m, H-4), 2.70 (2H, q, J = 7.5 Hz, H-7″), 3.38 (1H, t, J = 10.8, H-26), 3.47 (1H, dm, J = 10.8 Hz, H-26), 4.41 (1H, dd, J = 14.9, 7.5 Hz, H-16), 4.81 (1H, m, H-3), 5.43 (1H, d, J = 5.0 Hz, H-6), 7.25 (2H, d, J = 8.2 Hz, H-3″,5″), 7.59 (2H, d, J = 8.2 Hz, H-2″,6″). 13C NMR (101 MHz, CDCl3, δ, ppm): 14.5 (C-21), 15.0 (C-8″), 16.3 (C-18), 17.1 (C-27), 19.3 (C-19), 20.8 (C-11), 27.4 (C-2), 28.8 (C-24), 29.1 (C-7″), 30.2 (C-25), 31.3 (C-8), 31.4 (C-23), 31.8 (C-15), 32.0 (C-7), 36.7 (C-10), 36.8 (C-1), 37.6 (C-4), 39.6 (C-12), 40.2 (C-13), 41.6 (C-20), 49.8 (C-9), 56.4 (C-14), 62.0 (C-17), 66.8 (C-26), 77.3 (C-3), 80.8 (C-16), 87.3 (C-3′), 98.9 (C-4′), 109.3 (C-22), 116.2 (C-1″), 123.1 (C-6), 128.4 (C-3″,5″), 134.0 (C-2″,6″), 138.9 (C-5), 149.0 (C-4″), 158.8 (C-1′), 169.9 (C-2′). IR (KBr, ν, cm−1): 2197 (C≡C), 1736, 1676 (C=O), 1080, 1025, 1007 (C-O-C), 1605, 1508, 793, 756, 717 (C=C). HR-MS, m/z (Irel., %): 598 (1), 397 (37), 396 (67), 321 (28), 285 (36), 282 (100), 253 (21), 139 (90), 157 (47), 106 (39). Calcd. C39H50O5. m/z [M]+ 598.3653. Found, m/z: 598.3652.
Compound 6. (4S,5′R,6aR,6bS,8aS,8bR,9S,10R,11aS,12aS,12bS)-5′,6a,8a,9-Tetramet- hyl-1,3,3′,4,4′,5,5′,6,6a,6b,6′,7,8,8a,8b,9,11a,12,12a,12b-icosahydrospiro(naphtho[2′,1′:4,5]indeno[2,1-b]furan-10,2′-pyran)-4-yl 4-(4-methoxyphenyl)-2-oxobut-3-ynoate [(22R,25R)-spirost-5-en-3β-yl 4-(4-methoxy-phenyl)-2-oxobut-3-ynoate] (6). Yield 18%. Yellowish solid. M.p. 111 °C (decomp.). [α]D25 -92.9 (c 0.2, CHCl3). 1H NMR (400 MHz, CDCl3, δ, ppm): 0.74 (3H, d, J = 6.8 Hz, H-27), 0.77 (3H, s, H-19), 0.98 (3H, d, J = 7.0 Hz, H-21), 1.01 (1H, m, H-9), 1.08 (3H, s, H-18), 1.12–1.34 (4H, m, H-1,12,14,15), 1.41–1.96 (14H, m, H-1,2,7,8,11,11,12,17,20,23,23,24,24,25), 1.97–2.05 (3H, m, H-2,7,15), 2.49 (2H, m, H-4), 3.38 (1H, t, J = 10.8 Hz, H-26), 3.48 (1H, dm, J = 10.8 Hz, H-26), 3.87 (3H, s, H-OCH3), 4.41 (1H, dd, J = 14.5, 7.5 Hz, H-16), 4.81 (1H, m, H-3), 5.43 (1H, d, J = 5.0 Hz, H-6), 6.92 (2H, d, J = 8.6 Hz, H-3″,5″), 7.63 (2H, d, J = 8.6 Hz, H-2″,6″). 13C NMR (101 MHz, CDCl3, δ, ppm): 14.5 (C-21), 16.3 (C-18), 17.1 (C-27), 19.3 (C-19), 20.8 (C-11), 27.4 (C-2), 28.8 (C-24), 30.3 (C-25), 31.3 (C-8), 31.3 (C-23), 31.8 (C-15), 32.0 (C-7), 36.7 (C-10), 36.8 (C-1), 37.6 (C-4), 39.7 (C-12), 40.2 (C-13), 41.6 (C-20), 49.8 (C-9), 55.5 (OCH3), 56.4 (C-14), 62.0 (C-17), 66.8 (C-26), 77.2 (C-3), 80.8 (C-16), 87.8 (C-3′), 99.8 (C-4′), 109.3 (C-22), 110.8 (C-1″), 114.6 C-3″,5″), 123.1 (C-6), 136.1 (C-2″,6″), 139.0 (C-5), 158.9 (C-1′), 162.6 (C-4″), 169.7 (C-2′). IR (KBr, ν, cm−1): 2189 (C≡C), 1736, 1668 (C=O), 1080, 1066, 1025, 1007 (C-O-C), 1601, 1510, 800, 756, 733 (C=C). HR-MS, m/z (Irel., %): 600 (3), 398 (15), 397 (60), 396 (95), 283 (34), 282 (100), 159 (98), 139.1 (71), 69 (15). Calcd. C38H48O6, m/z [M]+ 600.3436. Found 600.3448.
Compound 7. (4S,5′R,6aR,6bS,8aS,8bR,9S,10R,11aS,12aS,12bS)-5′,6a,8a,9-Tetramet- hyl-1,3,3′,4,4′,5,5′,6,6a,6b,6′,7,8,8a,8b,9,11a,12,12a,12b-icosahydrospiro(naphtho[2′,1′:4,5]indeno[2,1-b]furan-10,2′-pyran)-4-yl 4-(4-fluorophenyl)-2-oxobut-3-ynoate [(22R,25R)-spirost-5-en-3β-yl 4-(4-fluorophenyl)-2-oxobut-3-ynoate] (7). Yield 46%. Yellowish crystal. M.p. 62 °C (decomp.). [α]D23—105.3 (c 0.2, CHCl3). 1H NMR (400 MHz, CDCl3, δ, ppm): 0.74 (3H, d, J = 6.8 Hz, H-27), 0.77 (3H, s, H-19), 0.98 (3H, d, J = 7.0 Hz, H-21), 1.02 (1H, m, H-9), 1.07 (3H, s, H-18), 1.13–1.22 (3H, m, H-1,12,14), 1.30 (1H, m, H-15), 1.43–1.54 (3H, m, H-11,11,24), 1.58–1.69 (6H, m, H-7,8,23,23,24,25), 1.71–1.81 (3H, m, H-2,12,17), 1.88–1.92 (2H, m, H-1,20), 1.95–2.05 (3H, m, H-2,7,15), 2.48 (2H, m, H-4), 3.38 (1H, t, J = 10.2 Hz, H-26), 3.47 (1H, dm, J = 10.2 Hz, H-26), 4.41 (1H, dd, J = 14.8, 7.4 Hz, H-16), 4.81 (1H, m, H-3), 5.43 (1H, s, H-6), 7.13 (2H, t, J = 8.6 Hz, H-3″,5″), 7.68 (2H, dd, J = 8.6, 5.4 Hz, H-2″,6″). 13C NMR (126 MHz, CDCl3, δ, ppm): 14.5 (C-21), 16.2 (C-18), 17.1 (C-27), 19.3 (C-19), 20.8 (C-11), 27.4 (C-2), 28.8 (C-24), 30.3 (C-25), 31.4 (C-8), 31.4 (C-23), 31.8 (C-15), 32.1 (C-7), 36.8 (C-10), 36.9 (C-1), 37.7 (C-4), 39.7 (C-12), 40.3 (C-13), 41.6 (C-20), 49.9 (C-9), 56.5 (C-14), 62.2 (C-17), 66.9 (C-26), 77.5 (C-3), 80.8 (C-16), 87.2 (C-3′), 96.7 (C-4′), 109.3 (C-22), 115.3, 115.4 (C-1″), 116.3, 116.5 (C-3″,5″), 123.2 (C-6), 136.1, 136.2 (C-2″,6″), 138.9 (C-5), 158.7 (C-1′), 164.6 (d, C-4″, JC-F = 255.7 Hz), 169.8 (C-2′). IR (KBr, ν, cm−1): 2191 (C≡C), 1740, 1680 (C=O), 1342, 1248, 1176, 1159 (C-F), 1082, 1066, 1024, 1007 (C-O-C), 1598, 1500, 815, 798, 760, 688 (C=C). HR-MS, m/z (Irel., %): 588 (2), 397 (40), 396 (78), 283 (31), 282 (100), 281 (16), 253 (18), 147 (18), 139 (54). Calcd. C37H45FO5, m/z [M]+ 588.3246. Found 588.3242.
Compound 8. (4S,5′R,6aR,6bS,8aS,8bR,9S,10R,11aS,12aS,12bS)-5′,6a,8a,9-Tetramet- hyl-1,3,3′,4,4′,5,5′,6,6a,6b,6′,7,8,8a,8b,9,11a,12,12a,12b-icosahydrospiro(naphtho[2′,1′:4,5]indeno[2,1-b]furan-10,2′-pyran)-4-yl 2-oxo-4-(m-tolyl)but-3-ynoate [(22R,25R)-spirost- 5-en-3β-yl 2-oxo-4-(m-tolyl) but-3-ynoate] (8). Yield 30%. Yellowish solid. M.p. 148 °C (decomp.). [α]D23—50.9 (0.3, CHCl3). 1H NMR (300 MHz, CDCl3, δ, ppm): 0.76 (3H, d, J = 6.8 Hz, H-27), 0.77 (3H, s, H-19), 0.98 (3H, d, J = 7.0 Hz, H-21), 1.01 (1H, m, H-9), 1.08 (3H, s, H-18), 1.12–1.34 (4H, m, H-1,12,14,15), 1.43–1.69 (9H, m, H-7,8,11,11,23,23,24,24,25), 1.71–1.81 (3H, m, H-2,12,17), 1.84–1.93 (2H, m, H-1,20), 1.94–2.05 (3H, m, H-2,7,15), 2.38 (3H, s, C7″H3), 2.49 (2H, m, H4,4), 3.38 (1H, t, J = 10.8 Hz, H-26), 3.47 (1H, dd, J = 10.6, 2.6 Hz, H-26), 4.41 (1H, dd, J = 14.9, 7.5 Hz, H-16), 4.82 (1H, m, H-3), 5.43 (1H, d, J = 4.9 Hz, H-6), 7.22 (1H, d, J = 7.8 Hz, H-4″), 7.28 (1H, c, H-2″), 7. 30 (1H, d, J = 7.8 Hz, H-6″), 7.49 (1H, t, J = 7.8 Hz, H-6″). 13C NMR (126 MHz, CDCl3, δ, ppm): 14.5 (C-21), 16.2 (C-18), 17.1 (C-27), 19.3 (C-19), 20.8 (C-11), 21.1 (C-7″), 27.4 (C-2), 28.8 (C-24), 30.3 (C-25), 31.4 (C-8), 31.4 (C-23), 31.8 (C-15), 32.1 (C-7), 36.7 (C-10), 36.9 (C-1), 37.7 (C-4), 39.7 (C-12), 40.3 (C-13), 41.6 (C-20), 49.9 (C-9), 56.5 (C-14), 62.2 (C-17), 66.8 (C-26), 77.4 (C-3), 80.8 (C-16), 87.0 (C-3′), 98.3 (C-4′), 109.2 (C-22), 119.0 (C-1″), 123.1 (C-6), 128.6 (C-5″), 130.9 (C-4″), 132.7 (C-2″), 134.2 (C-6″), 138.6 (C-3″), 139.1 (C-5), 158.8 (C-1′), 169.9 (C-2′). IR (KBr, ν, cm−1): 2195 (C≡C), 1740, 1678 (C=O), 1080, 1066, 1025, 1005 (C-O-C), 1596, 1500, 815, 785, 756, 733, 687 (C=C). HR-MS, m/z (Irel., %): 584 (1), 397 (32), 396 (65), 394 (27), 282 (49), 143 (100), 139 (96), 105 (16), 91 (17), 55 (19). Calcd. C38H48O5, m/z [M]+ 584.3496. Found 584.3495.
Four Component Reactions of (22R,25R)-Spirost-5-en-3β-yl 2-Chloro-2-Oxoacetate (2), Terminal Aryl Acetylenes (3a–e) and Phenylhydrazine Hydrochloride (9)
(a) A solution of diosgenin (800 mg, 1.93 mmol) in CHCl3 (10 mL) was added dropwise to a cold stirred solution of oxalyl chloride (980 mg (0.66 mL), 7.72 mmol) in CHCl3 (5 mL) in argon atmosphere at 0 °C for 1 h. The reaction mixture was stirred at room temperature for 3 h. The solvent was removed under reduced pressure, the residue was treated with 3ml of CHCl3 and additionally evaporated. This procedure was repeated three times for removing the trace of oxalyl chloride. The residue of 2 (975 mg) was dissolved in C6H6 (13 mL) and subsequently treated with aryl acetylene 3a–e (1.93 mmol), CuI (36 mg, 0.19 mmol) and Et3N (195 mg (0.27 mL), 1.93 mmol), under stirring in an argon flow. The reaction mixture was stirred at 40 °C for 12h (TLC), then phenylhydrazine hydrochloride 9 (185 mg, 1.29 mmol) and Et3N (130 mg, 0.18 mL, 1.29 mmol) were added and temperature was increased to 60 °C. After 12 h stirring in argon atmosphere (TLC) the solvent was removed and the residue was purified by column chromatography (petroleum ether–ether, 100:15) to afford the corresponding compounds 10–14. (b) A solution of diosgenin (800 mg, 1.93 mmol) in CHCl3 (10 mL) was added dropwise to a cold stirred solution of oxalyl chloride (980 mg (0.66 mL), 7.72 mmol) in CHCl3 (5 mL) in argon atmosphere at 0 °C for 1 h. The reaction mixture was additionally stirred at room temperature for 3 h. The solvent was removed under reduced pressure, the residue was treated with 3ml of CHCl3 and additionally evaporated. This procedure was repeated three times for removing the trace of oxalyl chloride and the residue was dissolved in C6H6 (13 mL) in an argon flow. The corresponding terminal aryl acetylene 3a–e (1.93 mmol), CuI (36 mg, 0.19 mmol), and Et3N (195 mg (0.27 mL), 1.93 mmol) were added. The reaction mixture was stirred at 40 °C for 12h (TLC), then compound 9 (185 mg,1.29 mmol), ethanol (7mL), and Et3N (130 mg, 0.18 mL, 1.29 mmol), were added and the temperature was increased to 60 °C. After 12 h stirring under argon atmosphere (TLC) the solvent was removed and the residue was subjected to column chromatography (eluent petroleum ether–ether, gradient from 20:1 to 100:15) with sequentional isolation of the steroidal (E)-alkynylhydrazones 15–19 and pyrazoles 10–14. (c) A solution of diosgenin 1 (800 mg, 1.93 mmol) in CHCl3 (10 mL) was added dropwise to a cold stirred solution of oxalyl chloride (980 mg (0.66 mL), 7.72 mmol) in CHCl3 (5 mL) in argon atmosphere at 0 °C for 1 h. The reaction mixture was stirred at room temperature for 3 h. The solvent was removed under reduced pressure, the residue was treated with 3ml of CHCl3 and additionally evaporated. This procedure was repeated three times for removing the trace of oxalyl chloride and the formed compound 2, aryl acetylene 3a (197 mg, 1.93 mmol), CuI (36 mg, 0.19 mmol) and Et3N (195 mg (0.27 mL), 1.93 mmol) was stirred in C6H6 (13 mL) in an argon flow at 40 °C for 12 h (TLC). The solvent was removed under reduced pressure, and the residue was treated with 2-methoxyethanol (15 mL). Then, phenylhydrazine hydrochloride 9 (223 mg, 1.54 mmol) and Et3N (156 mg (0.21 mL), 1.54 mmol) were added in an argon flow. The reaction mixture was stirred for 24 h at ambient temperature, then the solvent was evaporated. Column chromatography of the residue (eluent petroleum ether–ether, gradient from 100:3 to 100:15) afforded (Z)-alkynylhydrazone 20, (E)-alkynylhydrazone 15, pyrazole 21 and pyrazole 10.
Compound 10. (4S,5′R,6aR,6bS,8aS,8bR,9S,10R,11aS,12aS,12bS)-5′,6a,8a,9-Tetra- methyl-1,3,3′,4,4′,5,5′,6,6a,6b,6′,7,8,8a,8b,9,11a,12,12a,12b-icosahydrospiro(naphtho[2′,1′: 4,5]indeno[2,1-b]furan-10,2′-pyran)-4-yl 1,5-diphenyl-1H-pyrazole-3-carboxylate [(22R,25R)-spirost-5-en-3β-yl 1,5-diphenyl-1H-pyrazole-3-carboxylate] (10). Yield: 46% (method a), 9% (method b), 12% (method c). White solid. M.p. 207–210 °C (petroleum and diethyl ethers mixture). [α]D23 –67.3 (c 0.4, CHCl3). 1H NMR (600 MHz, CDCl3, δ, ppm): 0.78 (3H, s, H-19), 0.75 (3H, d, J = 6.8 Hz, H-27), 0.98 (3H, d, J = 7.0 Hz, H-21), 1.02 (1H, m, H-9), 1.08 (3H, s, H-18), 1.14 (1H, m, H-14), 1.22 (2H, m, H-1,12), 1.30 (1H, m, H-15), 1.42–1.52 (2H, m, H-11,24), 1.54–1.71 (7H, m, H-7,8,11,23,23,24,25), 1.76 (1H, dm, J = 12.5 Hz, H-12), 1.79–1.85 (2H, m, H-2,17), 1.89 (1H, m, H-20), 1.92 (1H, dt, J = 13.8, 3.7 Hz, H-1), 1.99–2.05 (3H, m, H-2,7,15), 2.53 (2H, m, H-4,4), 3.39 (1H, t, J = 10.7 Hz, H-26), 3.48 (1H, dm, J = 10.7 Hz, H-26), 4.42 (1H, dd, J = 15.0, 7.3 Hz, H-16), 4.95 (1H, m, H-3), 5.43 (1H, d, J = 4.8 Hz, H-6), 7.04 (1H, s, H-4′), 7.21–7.35 (10H, m, H-2″, 3″, 4″,5″, 6″,2‴,3‴,4‴,5‴,6‴). 13C NMR (151 MHz, CDCl3, δ, ppm): 14.5 (C-21), 16.3 (C-18), 17.1 (C-27), 19.4 (C-19), 20.8 (C-11), 27.1 (C-2), 28.8 (C-24), 30.3 (C-25), 31.3 (C-23), 31.4 (C-8), 31.8 (C-15), 32.1 (C-7), 36.8 (C-10), 37.0 (C-1), 38.1 (C-4), 39.7 (C-12), 40.3 (C-13), 41.6 (C-20), 49.9 (C-9), 56.4 (C-14), 62.1 (C-17), 66.8 (C-26), 74.7 (C-3), 80.8 (C-16), 109.2 (C-22), 109.9 (C-4′), 122.5 (C-6), 125.8 (2C), 128.3, 128.5, 128.6, 128.7, 128.9, 129.0, 129.2 (2C) (C-2″,6″,3″,5″,4″,2‴,6‴,3‴, 5‴,4‴), 129.6 (C-1″), 139.6 (C-1‴), 139.8 (C-5), 144.5, 144.6 (C-3′,5′), 161.9 (C=O). IR (KBr, ν, cm−1): 1718 (C=O), 1535, 1569 (C=N pyrazole), 1078, 1065, 1049, 1026, 1009 (C-O-C), 1599, 1502, 829, 796, 762, 698 (C=C). HR-MS, m/z (Irel., %): 660 (1), 396 (100), 265 (92), 282 (66), 139 (63), 247 (33), 397 (32), 266 (18), 283 (17), 324 (14). Calcd. C43H52N2O4, m/z [M]+. 660.3922. Found 660.3909.
Compound 11. (4S,5′R,6aR,6bS,8aS,8bR,9S,10R,11aS,12aS,12bS)-5′,6a,8a,9-Tetra- methyl-1,3,3′,4,4′,5,5′,6,6a,6b,6′,7,8,8a,8b,9,11a,12,12a,12b-icosahydrospiro(naphtho[2′,1′: 4,5]indeno[2,1-b]furan-10,2′-pyran)-4-yl 5-(4-ethylphenyl)-1-phenyl-1H-pyrazole-3-car- boxylate [(22R,25R)-spirost-5-en-3β-yl 5-(4-ethylphenyl)-1-phenyl-1H-pyrazole-3-carbo- xylate] (11). Yield: 53% (a), 13% (b). White needles. M.p. 177–180 °C. [α]D25—61.9 (c 0.2, CHCl3). 1H NMR (400 MHz, CDCl3, δ, ppm): 0.78 (3H, s, H-19), 0.76 (3H, d, J = 6.8 Hz, H-27), 0.98 (3H, d, J = 7.0 Hz, H-21), 1.02 (1H, m, H-9), 1.08 (3H, s, H-18), 1.24 (3H, t, J = 7.2 Hz, H-8″), 1.20–1.34 (4H, m, H-1,12,14,15), 1.44–1.68 (9H, m, H-7,8,11,11,23,23,24,24,25), 1.72–1.82 (3H, m, H-2,12,17), 1.87–1.94 (2H, m, H-1,20), 1.98–2.05 (3H, m, H-2,7,15), 2.52 (2H, m, H-4), 2.63 (2H, q, J = 7.2 Hz, H-7″), 3.39 (1H, t, J = 10.9 Hz, H-26), 3.48 (1H, dm, J = 10.9 Hz, H-26), 4.42 (1H, dd, J = 14.5, 7.4 Hz, H-16), 4.95 (1H, m, H-3), 5.43 (1H, d, J = 3.8 Hz, H-6), 7.01 (1H, s, H-4′), 7.13–7.26 (4H, m, H-2″,6″,3″, 5″), 7.28–7.35 (5H, m, H-2‴,6‴,3‴,5‴,4‴). 13C NMR (126 MHz, CDCl3, δ, ppm): 14.5 (C-21), 15.2 (C-8″), 16.3 (C-18), 17.1 (C-27), 19.4 (C-19), 20.8 (C-11), 27.7 (C-2), 28.5 (C-7″), 28.8 (C-24), 30.3 (C-25), 31.3 (C-23), 31.4 (C-8), 31.8 (C-15), 32.0 (C-7), 36.8 (C-10), 36.9 (C-1), 38.1 (C-4), 39.7 (C-12), 40.2 (C-13), 41.6 (C-20), 49.9 (C-9), 56.4 (C-14), 62.0 (C-17), 66.8 (C-26), 74.6 (C-3), 80.8 (C-16), 109.2 (C-22), 109.7 (C-4′), 122.4 (C-6), 125.8 (2C), 128.0, 128.2 (2C), 128.6 (2C), 128.9 (2C) (C-2″,6″,3″,5″,2‴,6‴,3‴,5‴, 4‴), 126.6 (C-1″), 139.6 (C-1‴), 139.7 (C-5), 144.5, 144.6 (C-3′,5′), 144.9 (C-4″), 161.9 (C=O). IR (KBr, ν, cm−1): 1714 (C=O), 1535, 1556 (C=N pyrazole), 1066, 1053, 1024, 1007 (C-O-C), 1599, 1502, 825, 796, 764, 710, 692 (C=C). HR-MS, m/z (Irel., %): 688 (1), 397 (18), 396 (52), 294 (20), 293 (100), 292 (25), 282 (39), 275 (14), 139 (23). Calcd. C45H56N2O4. m/z [M]+ 688.4149. Found 688.4154. X-ray structural analysis of compound 11: C45H56N2O4, M 688.92, monoclinic, P21, a 13.4005(8), b 7.8696(5), c 19.590(1) Å, β 108.795(3)º, V 1955.7(2) Å3, Z 2, Dcalcd 1.170g·cm−3, μ(Mo-Kα) 0.074 mm−1, F(000) 744, (θ 1.10–25.06°, completeness 99.7%), colorless, (0.64 × 0.20 × 0.05) mm3, transmission 0. 0.7514–0.8620. The intensities of 6917 independent reflections were measured (Rint 0.0393), 465 parameters, 7 restraints, R1 0.0557 (for 6319 observed I > 2σ(I)), wR2 = 0.1766 (all data), GOOF 1.067, largest diff. peak and hole 0.182 and -0.207 e.A−3. Crystallographic data for structure 11 have been deposited at the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC 2077784.
Compound 12. (4S,5′R,6aR,6bS,8aS,8bR,9S,10R,11aS,12aS,12bS)-5′,6a,8a,9-Tetra- methyl-1,3,3′,4,4′,5,5′,6,6a,6b,6′,7,8,8a,8b,9,11a,12,12a,12b-icosahydrospiro(naphtho[2′,1′: 4,5]indeno[2,1-b]furan-10,2′-pyran)-4-yl 5-(4-methoxyphenyl)-1-phenyl-1H-pyra- zole-3-carboxylate [(22R,25R)-spirost-5-en-3β-yl 5-(4-methoxyphenyl)-1-phenyl- 1H-pyrazole-3-carboxylate] (12). Yield: 54% (a), 17% (b). Yellowish needles. M.p. 223–224 °C (decomp.). [α]D22 -61.9 (c 0.2, CHCl3). 1H NMR (400 MHz, CDCl3, δ, ppm): 0.77 (3H, s, H-19), 0.76 (3H, d, J = 6.8 Hz, H-27), 0.98 (3H, d, J = 6.9 Hz, H-21), 1.01 (1H, m, H-9), 1.08 (3H, c, H-18), 1.12–1.34 (4H, m, H-1,12,14,15), 1.43–1.68 (9H, m, H-7,8,11,11,23, 23,24,24,25), 1.71–1.82 (3H, m, H-2,12,17), 1.85–1.93 (2H, m, H-1,20), 1.97–2.05 (3H, m, H-2,7,15), 2.52 (2H, m, H-4), 3.39 (1H, t, J = 10.9 Hz, H-26), 3.48 (1H, dm, J = 10.9, H-26), 3.77 (3H, s, OCH3), 4.42 (1H, dd, J = 15.0, 7.7 Hz, H-16), 4.94 (1H, m, H-3), 5.42 (1H, d, J = 5.0, H-6), 6.78 (2H, d, J = 8.7 Hz, H-3″,5″), 6.94 (1H, s, H-4′), 7.12 (2H, d, J = 8.7 Hz, H-2″,6″), 7.26–7.35 м (5H, H-2‴,3‴,4‴,5‴,6‴). 13C NMR (126 MHz, CDCl3, δ, ppm): 14.5 (C-21), 16.3 (C-18), 17.1 (C-27), 19.4 (C-19), 20.8 (C-11), 27.7 (C-2), 28.8 (C-24), 30.3 (C-25), 31.3 (C-23), 31.4 (C-8), 31.8 (C-15), 32.0 (C-7), 36.8 (C-10), 36.9 (C-1), 38.0 (C-4), 39.7 (C-12), 40.2 (C-13), 41.6 (C-20), 49.9 (C-9), 55.2 (OCH3), 56.4 (C-14), 62.0 (C-17), 66.8 (C-26), 74.6 (C-3), 80.8 (C-16), 109.2 (C-22), 109.4 (C-4′), 113.9 (C-3″,5″), 121.92 (C-1″), 122.4 (C-6), 125.8 (2C), 128.2, 128.9 (2C) (C-2‴,6‴,3‴,5‴,4‴), 130.0 (C-2″,6″), 139.6 (C-1‴), 139.7 (C-5), 144.4, 144.5 (C-3′,5′), 159.8 (C-4″), 161.9 (C=O). IR (KBr, ν, cm−1): 1738 (C=O), 1533, 1552, 1578 (C=N pyrazole), 1066, 1051, 1028, 1007 (C-O-C), 1612, 1500, 812, 796, 777, 762, 692 (C=C). HR-MS, m/z (Irel., %): 690 (1), 397 (12), 396 (41), 296 (14), 295 (100), 294 (53), 282 (32), 277 (28), 139 (39). Calcd. C44H54N2O5, m/z [M]+ 690.4027. Found 690.4094. X-ray structural analysis of compound 12: C44H54N2O5, M 690.89, monoclinic, P21, a 13.3267(6), b 7.8178(3), c 19.2083(9) Å, β 108.337(2)º, V 1899.6(1) Å3, Z 2, Dcalcd 1.208 g·cm−3, μ(Mo-Kα) 0.078 mm−1, F(000) 744, (θ 2.23–25.02°, completeness 99.9%), colorless, (0.64 × 0.20 × 0.05) mm3, transmission 0.8295–0.8620. The intensities of 6691 independent reflections were measured (Rint 0.0322), 465 parameters, R1 0.0355 (for 5873 observed I > 2σ(I)), wR2 = 0.1119 (all data), GOOF 1.091, largest diff. peak and hole 0.167 and -0.197 e.A−3. Crystallographic data for structure 12 have been deposited at the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC 2077782.
Compound 13. (4S,5′R,6aR,6bS,8aS,8bR,9S,10R,11aS,12aS,12bS)-5′,6a,8a,9-Tetra- methyl-1,3,3′,4,4′,5,5′,6,6a,6b,6′,7,8,8a,8b,9,11a,12,12a,12b-icosahydrospiro(naphtho[2′,1′: 4,5]indeno[2,1-b]furan-10,2′-pyran)-4-yl 5-(4-fluorophenyl)-1-phenyl-1H-pyrazole-3- carboxylate [(22R,25R)-spirost-5-en-3β-yl 5-(4-fluorophenyl)-1-phenyl-1H-pyrazole- 3-carboxylate] (13). Yield: 48% (a), 69% (b). Yellowish crystals. M.p. 223 °C (decomp.). [α]D22—52.2 (0.23, CHCl3). 1H NMR (400 MHz, CDCl3, δ, ppm): 0.77 (3H, s, H-19), 0.75 (3H, d, J = 7.0 Hz, H-27),0.98 (3H, d, J = 6.9 Hz, H-21), 1.01 (1H, m, H-9), 1.08 (3H, s, H-18), 1.11–1.34 (4H, m, H-1,12,14,15), 1.44–1.68 (9H, m, H-7,8,11,11,23,23,24,24,25), 1.71–1.81 (3H, m, H-2,12,17), 1.85–1.94 (2H, m, H-1,20), 1.97–2.05 (3H, m, H-2,7,15), 2.52 (2H, m H-4), 3.39 (1H, t, J = 10.9 Hz, H-26), 3.48 (1H, dm, J = 10.9 Hz, H-26), 4.42 (1H, dd, J = 14.9, 7.4 Hz, H-16), 4.95 (1H, m, H-3), 5.43 (1H, d, J = 4.0 Hz, H-6), 6.98–7.02 (3H, m, H-4′,3″,5″), 7.18 (2H, dd, J = 8.5, 5.4 Hz, H-2″,6″), 7.29–7.36 (5H, m, H-2‴,6‴,3‴,5‴,4‴). 13C NMR (101 MHz, CDCl3,) δ, ppm): 14.5 (C-21), 16.3 (C-18), 17.1 (C-27), 19.4 (C-19), 20.8 (C-11), 27.7 (C-2), 28.76 (C-24), 30.26 (C-25), 31.35 (C-23), 31.39 (C-8), 31.81 (C-15), 32.04 (C-7), 36.8 (C-10), 36.98 (C-1), 38.04 (C-4), 39.69 (C-12), 40.23 (C-13), 41.57 (C-20), 49.89 (C-9), 56.4 (C-14), 62.03 (C-17), 66.80 (C-26), 74.69 (C-3), 80.76 (C-16), 109.24 (C-22), 109.91 (C-4′), 115.6, 115.8 (C-3″,5″), 122.5 (C-6), 125.7 (C-1″), 125.8 (C-2‴,6‴), 128.4, (C-3‴,5‴), 129.1 (C-4‴), 130.5, 130.6 (C-2″,6″), 139.3 (C-1‴), 139.7 (C-5), 144.1, 144.6 (C-3′,5′), 162.7 (C-4″, JC-F = 257.8 Hz), 161.8 (C=O). IR (KBr, ν, cm−1): 1718 (C=O), 1531, 1554 (C=N pyrazole), 1342, 1227, 1194, 1159 (C-F), 1068, 1051, 1026, 1007 (C-O-C), 1610, 1500, 816, 796, 777, 758, 692 (C=C). HR-MS, m/z (Irel, %): 678 (1), 397 (26), 396 (84), 283 (25), 282 (100), 265 (33), 139 (95), 69 (26). Calcd.C43H51FN2O4, m/z [M]+ 678.3827. Found 678.3828.
Compound 14. (4S,5′R,6aR,6bS,8aS,8bR,9S,10R,11aS,12aS,12bS)-5′,6a,8a,9-Tetra- methyl-1,3,3′,4,4′,5,5′,6,6a,6b,6′,7,8,8a,8b,9,11a,12,12a,12b-icosahydrospiro(naphtho[2′,1′: 4,5]indeno[2,1-b]furan-10,2′-pyran)-4-yl 1-phenyl-5-(m-tolyl)-1H-pyrazole-3-carboxylate [(22R,25R)-spirost-5-en-3β-yl 1-phenyl-5-(m-tolyl)-1H-pyrazole-3-carboxylate] (14). Yield: 60% (a), 39% (b). Red needles. M.p. 101 °C (decomp.). [α]D23—56.6 (c 0.2, CHCl3). 1H NMR (300 MHz, CDCl3, δ, ppm): 0.78 (3H, s, H-19), 0.75 (3H, d, J = 7.0 Hz, H-27), 0.98 (3H, d, J = 6.9 Hz, H-21), 1.02 (1H, m, H-9), 1.08 (3H, s, H-18), 1.12–1.34 (4H, m, H-1,12,14,15), 1.44–1.69 (9H, m, H-7,8,11,11,23,23,24,24,25), 1.72–1.83 (3H, m, H-2,12,17), 1.85–1.94 (2H, m, H-1,20), 1.98–2.05 (3H, m, H-2,7,15), 2.29 (3H, s, H-7″) 2.53 (2H, m, H-4), 3.39 (1H, t, J = 10.9 Hz, H-26), 3.48 (1H, dm, J = 10.9 Hz, H-26), 4.42 (1H, dd, J = 14.9, 7.1 Hz, H-16), 4.95 (1H, m, H-3), 5.43 (1H, J = 4.9 Hz, H-6), 6.87 (1H, J = 7.3 Hz, H-6″), 7.02 (1H, s, H-4′), 7.08 (1H, br.s, H-2″), 7.09–7.21 (2H, m, H-4″,5″), 7.29–7.39 (5H, m, H-2‴,6‴,3‴,5‴,4‴). 13C NMR (75 MHz, CDCl3, δ, ppm): 14.5 (C-21), 16.3 (C-18), 17.1 (C-27), 19.4 (C-19), 20.8 (C-11), 21.3 (C-7″), 27.7 (C-2), 28.8 (C-24), 30.3 (C-25), 31.4 (C-23), 31.4 (C-8), 31.8 (C-15), 32.1 (C-7), 36.8 (C-10), 37.0 (C-1), 38.1 (C-4), 39.7 (C-12), 40.2 (C-13), 41.6 (C-20), 49.9 (C-9), 56.4 (C-14), 62.1 (C-17), 66.8 (C-26), 74.6 (C-3), 80.8 (C-16), 109.3 (C-22), 109.9 (C-4′), 115.68 (C-2″), 122.45 (C-6), 125.74, 125.83, 128.23, 128.3, 128.87, 129.38 (C-4″,5″,6″,2‴,6‴,3‴,5‴, 4‴), 129.49 (C-3″), 138.29 (C-1″), 139.6 (C-1‴), 139.8 (C-5), 144.5, 144.7 (C-3′,5′), 161.9 (C=O). IR (KBr, ν, cm−1): 1716 (C=O), 1528, 1562 (C=N pyrazole), 1070, 1053, 1026, 1007 (C-O-C), 1599, 1500, 825, 777, 762, 700 (C=C). HR-MS, m/z (Irel., %): 674 (0.4), 396 (54), 397 (28), 283 (20), 282 (74), 143 (77), 139 (100), 105 (23), 91 (26), 69 (23). Calcd. C44H54N2O4, m/z [M]+ 674.4078. Found 674.4073.
Compound 15. (4S,5′R,6aR,6bS,8aS,8bR,9S,10R,11aS,12aS,12bS)-5′,6a,8a,9-Tetra- methyl-1,3,3′,4,4′,5,5′,6,6a,6b,6′,7,8,8a,8b,9,11a,12,12a,12b-icosahydrospiro(naphtho[2′,1′: 4,5]indeno[2,1-b]furan-10,2′-pyran)-4-yl (E)-4-phenyl-2-(2-phenylhydrazineylidene)- but-3-ynoate [(22R,25R)-spirost-5-en-3β-yl (E)-4-phenyl-2-(2-phenylhydrazineylidene)- but-3-ynoate] (15). Yield 35% (b), 47% (c). Yellow needles. M.p. 165 °C (decomp.). [α]D23 -59.0 (c 0.27, CHCl3). 1H NMR (300 MHz, CDCl3, δ, ppm): 0.78 (3H, s, H-19), 0.77 (3H, d, J = 6.8 Hz, H-27), 0.99 (3H, d, J = 6.7 Hz, H-21), 1.04 (1H, m, H-9), 1.10 (3H, s, H-18), 1.14–1.22 (2H, m, H-1,14), 1.24–1.36 (2H, m, H-12,15), 1.43–1.97 (14H, m, H-1,2,7,8,11,11,12, 17,20,23,23,24,24,25), 1.99–2.08 (3H, m, H-2,7,15), 2.52 (2H, m, H-4,4), 3.39 (1H, t, J = 10.8 Hz, H-26), 3.49 (1H, dd, J = 10.8, 3.1 Hz, H-26), 4.42 (1H, dd, J = 14.8, 7.6 Hz, H-16), 4.83 (1H, m, H-3), 5.43 (1H, d, J = 4.4 Hz, H-6). 7.05 (1H, t, J = 7.0 Hz, H-4″), 7.28–7.45, 7.59–7.62 (9H, m, H-2″,6″,3″,5″,2‴,6‴,3‴,5‴,4‴), 9.09 (1H, s, NH). 13C NMR (75 MHz, CDCl3, δ, ppm): 14.5 C-21), 16.3 (C-18), 17.1 (C-27), 19.4 (C-19), 20.8 (C-11), 27.1 (C-2), 28.8 (C-24), 30.3 (C-25), 31.3 (C-23), 31.4 (C-8), 31.8 (C-15), 32.1 (C-7), 36.8 (C-10), 36.9 (C-1), 38.1 (C-4), 39.7 (C-12), 40.2 (C-13), 41.5 (C-20), 49.9 (C-9), 56.4 (C-14), 62.1 (C-17), 66.8 (C-26), 75.3 (C-3), 77.7 (C-3′), 80.8 (C-16), 104.8 (C-4′), 109.2 (C-22), 114.8 (C-4″), 117.8 (C-2′), 121.4 (C-1″), 122.5 (C-6), 123.4 (C-4‴), 128.6, 129.4, 129.6, 131.9 (2C-2″,6″,3″,5″,2‴,6‴,3‴,5‴), 139.74 (C-5), 141.8 (C-1‴), 162.3 (C-1′). IR (KBr, ν, cm−1): 3282 (NH), 2181 (C≡C), 1711 (C=O), 1531 (C=N), 1070, 1049, 1028, 1009 (C-O-C), 1603, 1502, 812, 802, 754, 733, 716, 690 (C=C). HR-MS, m/z (Irel, %): 660 (10), 139 (100), 282 (77), 396 (77), 193 (50), 265 (48), 397 (37), 264 (33), 115 (32), 324 (30). Found, m/z: 660.3913 [M]+. C43H52N2O4. Calculated, m/z: 660.3922. X-ray structural analysis of compound 15: C43H52N2O4, M 660.87, orthorhombic, P212121, a 6.859(1), b 11.538(2), c 47.200(7) Å, V 3735.1(9) Å3, Z 4, Dcalcd 1.175 g·cm−3, μ(Mo-Kα) 0.075 mm−1, F(000) 1424, (θ 0.86–25.25°, completeness 99.5%), colorless, (0.75 × 0.14 × 0.05) mm3, transmission 0.7744–0.8620. The intensities of 6536 independent reflections were measured (Rint 0.0647), 446 parameters, R1 0.0484 (for 5539 observed I > 2σ(I)), wR2 = 0.1532 (all data), GOOF 0.947, largest diff. peak and hole 0.226 and -0.236 e.A−3. Crystallographic data for structure 15 have been deposited at the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC 2077783.
Compound 16. (4S,5′R,6aR,6bS,8aS,8bR,9S,10R,11aS,12aS,12bS)-5′,6a,8a,9-Tetra- methyl-1,3,3′,4,4′,5,5′,6,6a,6b,6′,7,8,8a,8b,9,11a,12,12a,12b-icosahydrospiro(naphtho[2′,1′: 4,5]indeno[2,1-b]furan-10,2′-pyran)-4-yl (E)-4-(4-ethylphenyl)-2-(2-phenylhydrazineyli- dene)-but-3-ynoate [(22R,25R)-spirost-5-en-3β-yl [(E)-4-(4-ethylphenyl)-2-(2-phenylhyd- razineylidene)-but-3-ynoate] (16). Yield 37% (b). Yellow needles. M.p. 98 °C (decomp.). [α]D20 -49.6 (c 0.2, CHCl3). 1H NMR (600 MHz, CDCl3, δ, ppm): 0.78 (3H, s, H-19), 0.76 (3H, d, J = 6.8 Hz, H-27), 0.99 (3H, d, J = 7.0, H-21), 1.02 (1H, m, H-9), 1.10 (3H, s, H-18), 1.14 (1H, m, H-14), 1.22 (2H, m, H-1,12), 1.27 (3H, t, J = 7.7 Hz, H-C8″H3), 1.30 (1H, m, H-15), 1.48 (2H, m, H-11,24), 1.55–1.77 (7H, m, H-7,8,11,23,23,24,25), 1.75–1.77 (3H, m, H-2,12,17), 1.86–1.93 (2H, m, H-1,20), 1.99–2.05 (3H, m, H-2,7,15), 2.51 (2H, m, H-4), 2.70 (2H, q, J = 7.7 Hz, H-7″), 3.39 (1H, t, J = 11.0 Hz, H-26), 3.49 (1H, dm, J = 11.0 Hz, H-26), 4.43 (1H, dd, J = 15.4, 7.7 Hz, H-16), 4.83 (1H, m, H-3), 5.42 (1H, d, J = 5.1 Hz, H-6), 7.05 (1H, t, J = 7.3 Hz, H-4‴), 7.25 (2H, d, J = 8.1 Hz, H-3″,5″), 7.29 (2H, d, J = 7.7 Hz, H-2‴,6‴), 7.34 (2H, t, J = 7.3 Hz, H-3‴,5‴), 7.51 (2H, d, J =8.1 Hz, H-2″,6″), 9.08 (1H, s, NH). 13C NMR (151 MHz, CDCl3, δ, ppm): 14.5 (C-21), 15.3 (C-8″), 16.3 (C-18), 17.1 (C-27), 19.4 (C-19), 20.8 (C-11), 27.7 (C-2), 28.8 (C-24), 28.9 (C-7″), 30.3 (C-25), 31.3 (C-23), 31.5 (C-8), 31.8 (C-15), 32.1 (C-7), 36.8 (C-10), 37.0 (C-1), 38.1 (C-4), 39.7 (C-12), 40.3 (C-13), 41.6 (C-20), 49.9 (C-9), 56.4 (C-14), 62.1 (C-17), 66.8 (C-26), 75.3 (C-3), 77.3 (C-3′), 80.8 (C-16), 105.2 (C-4′), 109.3 (C-22), 114.8 (C-2‴,6‴), 118.0 (C-2′), 118.5 (C-1″), 122.4 (C-6), 123.2 (C-4‴), 128.2 (C-3″,5″), 129.4 (C-3‴,5‴), 131.9 (C-2″,6″), 139.8 (C-5), 141.9 (C-1‴), 146.4 (C-4″), 162.4 (C-1′). IR (KBr, ν, cm−1): 3279 (NH), 2187 (C≡C), 1711 (C=O), 1559 (C=N), 1068, 1051, 1028, 1007 (C-O-C), 1602, 1510, 831, 800, 752, 710, 690 (C=C). HR-MS, m/z (Irel., %): 688 (1), 396 (50), 294 (20), 293 (100), 292 (24), 282 (46), 275 (56), 139 (77), 69 (33), 55 (20). Calcd. C45H56N2O4, m/z [M]+ 688.4235. Found 688.4233.
Compound 17. (4S,5′R,6aR,6bS,8aS,8bR,9S,10R,11aS,12aS,12bS)-5′,6a,8a,9-Tetra- methyl-1,3,3′,4,4′,5,5′,6,6a,6b,6′,7,8, 8a,8b,9,11a,12,12a,12b-icosahydrospiro[naphtho(2′,1′: 4,5]indeno[2,1-b]furan-10,2′-pyran)-4-yl (E)-4-(4-methoxyphenyl)-2-(2-phenylhydrazine- ylidene)but-3-ynoate [(22R,25R)-spirost-5-en-3β-yl (E)-4-(4-methoxyhenyl)-2-(2-phenyl- hydrazineylidene)but-3-ynoate] (17). Yield 38% (b). Yellow solid. M.p. 111 °C (decomp.). [α]D22 -53.1 (c 0.2, CHCl3). 1H NMR (600 MHz, CDCl3, δ, ppm): 0.76 (3H, s, H-19), 0.77 (3H, d, J = 7.0 Hz, H-27), 0.98 (3H, d, J = 7.0 Hz, H-21), 1.02 (1H, m, H-9), 1.09 (3H, s, H-18), 1.14 (1H, m, H-14), 1.21 (2H, m, H-1,12), 1.30 (1H, m, H-15), 1.48 (2H, m, H-11,24), 1.54–1.71 (7H, m, H-7,8,11,23,23,24,25), 1.74–1.81 (3H, m, H-2,12,17), 1.86–1.93 (2H, m, H-1,20), 1.99–2.06 (3H, m, H-2,7,15), 2.52 (2H, m, H-4), 3.39 (1H, t, J = 10.8 Hz, H-26), 3.48 (1H, dm, J = 10.8 Hz, H-26), 3.86 (3H, s, H-OCH3), 4.42 (1H, dd, J =15.0, 7.3 Hz, H-16), 4.82 (1H, m, H-3), 5.42 (1H, d, J = 5.1 Hz, H-6), 6.93 (2H, d, J = 8.8 Hz, H-3″,5″), 7.04 (1H, t, J = 7.3 Hz, H-4‴), 7.28 (2H, d, J = 8.2 Hz, H-2‴,6‴), 7.34 (2H, dd, J = 8.2, 7.3 Hz, H-3‴,5‴), 7.53 (2H, d, J = 8.8 Hz, H-2″,6″), 9.06 (1H, s, NH). 13C NMR (151 MHz, CDCl3, δ, ppm): 14.5 (C-21), 16.3 (C-18), 17.1 (C-27), 19.4 (C-19), 20.8 (C-11), 27.7 (C-2), 28.8 (C-24), 30.3 (C-25), 31.3 (C-23), 31.5 (C-8), 31.8 (C-15), 32.1 (C-7), 36.8 (C-10), 37.0 (C-1), 38.1 (C-4), 39.7 (C-12), 40.2 (C-13), 41.6 (C-20), 49.9 (C-9), 55.4 (OCH3), 56.4 (C-14), 62.1 (C-17), 66.8 (C-26), 75.3 (C-3), 76.8 (C-3′), 80.8 (C-16), 105.2 (C-4′), 109.2 (C-22), 113.4 (C-1″), 114.3 (C-3″,5″), 114.7 (C-2‴,6‴), 118.2 (C-2′), 122.4 (C-6), 123.1 (C-4‴), 129.4 (C-3‴,5‴), 133.5 (C-2″,6″), 139.8 (C-5), 141.9 (C-1‴), 160.7 (C-4″), 162.4 (C-1′). IR (KBr, ν, cm−1): 3280 (NH), 2191 (C≡C), 1711 (C=O), 1527 (C=N), 1068, 1051, 1026, 1007 (C-O-C), 1603, 1500, 833, 797, 785, 750, 710, 690 (C=C). HR-MS, m/z (Irel., %): 690 (1), 397 (14), 396 (46), 295 (100), 294 (59), 277 (34), 282 (43), 139 (40), 105 (13), 91 (14). Calcd. C44H54N2O5, m/z [M]+ 690.4027. Found, m/z: 690.4042.
Compound 18. (4S,5′R,6aR,6bS,8aS,8bR,9S,10R,11aS,12aS,12bS)-5′,6a,8a,9-Tetra- methyl-1,3,3′,4,4′,5,5′,6,6a,6b,6′,7,8,8a,8b,9,11a,12,12a,12b-icosahydrospiro(naphtho[2′,1′: 4,5]indeno[2,1-b]furan-10,2′-pyran)-4-yl (E)-4-(4-fluorophenyl)-2-(2-phenylhydrazineyli- dene)but-3-ynoate [(22R,25R)-spirost-5-en-3β-yl (E)-4-(4-fluorophenyl)-2-(2-phenylhyd- razineylidene)but-3-ynoate] (18). Yield 27% (b). Yellow solid. M.p. 109 °C (decomp.). [α]D24—46.0 (c 0.2, CHCl3). 1H NMR (400 MHz, CDCl3, δ, ppm): 0.78 (3H, s, H-19), 0.77 (3H, d, J = 6.8 Hz, H-27),0.98 (3H, d, J = 7.0 Hz, H-C21H3), 1.02 (1H, m, H-9), 1.10 (3H, s, H-18), 1.13–1.35 (4H, m, H-1,12,14,15), 1.44–1.69 (9H, m, H-7,8,11,11,23,23,24,24,25), 1.72–1.82 (3H, m, H-2,12,17), 1.85–1.94 (2H, m, H-1,20), 1.98–2.06 (3H, m, H-2,7,15), 2.50 (2H, m, H-4), 3.39 (1H, t, J = 10.8 Hz, H-26), 3.48 (1H, dm, J = 10.8 Hz, H-26), 4.42 (1H, dd, J = 15.0, 7.5 Hz, H-16), 4.82 (1H, m, H-3), 5.42 (1H, d, J = 4.6 Hz, H-6), 7.08 (1H, d, J = 7.4 Hz, H-4‴), 7.12 (2H, t, J = 8.6 Hz, H-3″,5″), 7.29 (2H, d, J =7.9, H-2‴,6‴), 7.35 (2H, dd, J = 7.9, 7.3 Hz, H-3‴,5‴), 7.59 (2H, dd, J = 8.9, 5.4 Hz, H-2″,6″), 9.05 (1H, s, NH). 13C NMR (101 MHz, CDCl3, δ, ppm): 14.5 (C-21), 16.3 (C-18), 17.1 (C-27), 19.4 (C-19), 20.8 (C-11), 27.7 (C-2), 28.7 (C-24), 30.3 (C-25), 31.3 (C-23), 31.4 (C-8), 31.8 (C-15), 32.0 (C-7), 36.8 (C-10), 36.9 (C-1), 38.0 (C-4), 39.7 (C-12), 40.2 (C-13), 41.6 (C-20), 49.9 (C-9), 56.4 (C-14), 62.0 (C-17), 66.8 (C-26), 75.4 (C-3), 77.5 (C-3′), 80.8 (C-16), 103.6 (C-4′), 109.3 C-22), 114.8 (C-2‴,6‴), 115.9, 116.2 (C-2″,6″), 117.5 (C-1″), 117.6 (C-2′), 122.5 (C-6), 123.3 (C-4‴), 129.4 (C-3‴,5‴), 133.9, 134.0 (C-3″,5″), 139.7 (C-5), 141.7 (C-1‴), 162.6 (C-4″, JC-F = 255.2 Hz), 162.3 (C-1′). IR (KBr, ν, cm−1): 3280 (NH), 2195 (C≡C), 1711 (C=O), 1529 (C=N), 1336, 1271, 1191, 1155 (C-F), 1068, 1051, 1026, 1007 (C-O-C), 1603, 1508, 816, 798, 752, 708, 690 (C=C). HR-MS, m/z (Irel., %): 678 (4), 397 (23), 396 (57), 395 (15), 394 (26), 283 (18), 282 (62), 211 (27), 139 (100), 115 (16). Calcd. C43H51FN2O4, m/z [M]+ 678.3827. Found, m/z: 678.3830.
Compound 19. (4S,5′R,6aR,6bS,8aS,8bR,9S,10R,11aS,12aS,12bS)-5′,6a,8a,9-Tetra- methyl-1,3,3′,4,4′,5,5′,6,6a,6b,6′,7,8,8a,8b,9,11a,12,12a,12b-icosahydrospiro[naphtho[2′,1′: 4,5]indeno[2,1-b]furan-10,2′-pyran]-4-yl (E)-2-(2-phenylhydrazineylidene)-4-(m-tolyl)- but-3-ynoate [(22R,25R)-spirost-5-en-3β-yl (E)-2-(2-phenylhydrazineyli- dene)-4-(m-tolyl)but-3-ynoate] (19). Yield 37% (b). Yellow solid. M.p. 93 °C (decomp.). [α]D25 -64.6 (c 0.2, CHCl3). 1H NMR (500 MHz, CDCl3, δ, ppm): 0.78 (3H, s, H-19), 0.77 (3H, d, J = 6.7 Hz, H-27), 0.98 (3H, d, J = 7.0, H-21), 1.02 (1H, m, H-9), 1.10 (3H, s, H-18), 1.14 (1H, m, H-14), 1.21 (2H, m, H-1,12), 1.30 (1H, m, H-15), 1.48 (2H, m, H-11,24), 1.55–1.72 (7H, m, H-7,8,11,23,23,24,25), 1.75–1.81 (3H, m, H-2,12,17), 1.89 (1H, q, J = 7.0 Hz, H-20), 1.92 (1H, dt, J = 13.6, 3.7 Hz, H-1), 1.99–2.06 (3H, m, H-2,7,15), 2.40 (3H, s, H-7″), 2.52 (2H, m, H-4), 3.39 (1H, t, J = 10.8 Hz, H-26), 3.48 (1H, dm, J = 10.8 Hz, H-26), 4.43 (1H, dd, J = 15.0, 7.3 Hz, H-16), 4.83 (1H, m, H-3), 5.42 (1H, d, J = 5.1 Hz, H-6), 7.05 (1H, t, J = 7.4 Hz, H-4‴), 7.25 (1H, d, J = 7.6 Hz, H-4″), 7.29–7.36 (5H, m, H-5″,2‴,6‴,3‴,5‴), 7.40 (1H, d, J = 7.9 Hz, H-6″), 7.42 (1H, s, H-2″), 9.09 (1H, s, NH). 13C NMR (126 MHz, CDCl3, δ, ppm): 14.5 (C-21), 16.3 (C-18), 17.1 (C-27), 19.4 (C-19), 20.8 (C-11), 21.2 (C-7″), 27.7 (C-2), 28.8 (C-24), 30.3 (C-25), 31.3 (C-23), 31.4 (C-8), 31.8 (C-15), 32.2 (C-7), 36.8 (C-10), 37.0 (C-1), 38.1 (C-4), 39.7 (C-12), 40.4 (C-13), 41.6 (C-20), 49.9 (C-9), 56.4 (C-14), 62.1 (C-17), 66.8 (C-26), 75.3 C-3), 77.4 (C-3′), 80.8 (C-16), 105.1 (C-4′), 109.2 (C-22), 114.8 (C-2‴,6‴), 117.9 (C-2′), 121.2 (C-3″), 122.4 (C-6), 123.2 (C-4‴), 128.5 (C-4″), 129.0 (C-5″), 129.4 (C-3‴,5‴), 130.6 (C-2″), 132.4 (C-6″), 138.4 (C-1″), 139.8 (C-5), 141.9 (C-1‴), 162.4 (C-1′). IR (KBr, ν, cm−1): 3281 (NH), 2189 (C≡C), 1711 (C=O), 1527 (C=N), 1068, 1051, 1026, 1007 (C-O-C), 1603, 1502, 835, 814, 783, 752, 688 (C=C). HR-MS, m/z (Irel., %): 674 (0.4), 397 (28), 396 (54), 283 (20), 282 (74), 143 (75), 139 (100), 105 (23), 91 (26), 69 (23). Calcd. C44H54N2O4. m/z [M]+ 674.4078. Found, m/z: 674.4073.
Compound 20. (4S,5′R,6aR,6bS,8aS,8bR,9S,10R,11aS,12aS,12bS)-5′,6a,8a,9-Tetra- methyl-1,3,3′,4,4′,5,5′,6,6a,6b,6′,7,8,8a,8b,9,11a,12,12a,12b-icosahydrospiro(naphtho[2′,1′: 4,5]indeno[2,1-b]furan-10,2′-pyran)-4-yl (Z)-4-phenyl-2-(2-phenylhydrazineylidene)but- 3-ynoate [(22R,25R)-spirost-5-en-3β-yl (Z)-4-phenyl-2-(2-phenylhydrazineylidene)but- 3-ynoate] (20). Yield 7% (c). Yellow crystals. M.p. 146 °C (decomp.). [α]D25 -93.8 (c 0.2, CHCl3). 1H NMR (300 MHz, CDCl3, δ, ppm): 0.77 (3H, s, H-19), 0.76 (3H, d, J = 6.8 Hz, H-27), 0.97 (3H, d, J = 6.8 Hz, H-21), 1.01 (1H, m, H-9), 1.07 (3H, s, H-18), 1.11–1.34 (4H, m, H-1,12,14,15), 1.41–2.02 (17H, m, H-1,2,2,7,7,8,11,11,12,15, 17,20,23,23,24, 24,25), 2.47 (2H, m, H-4), 3.36 (1H, t, J = 10.7 Hz, H-26), 3.45 (1H, dd, J = 10.7, 3.4 Hz, H-26), 4.39 (1H, dd, J = 14.8, 7.5 Hz, H-16), 4.72 (1H, m, H-3), 5.43 (1H, d, J = 4.1 Hz, H-6), 7.03 (1H, t, J = 6.7 Hz, H-4″), 7.24–7.34 (7H, m, H-2″,6″,2‴,4‴,3‴,5‴,6‴), 7.50–7.53 (2H, m, H-3″,5″), 12.8 (1H, s, NH). 13C NMR (126 MHz, CDCl3, δ, ppm: 14.5 (C-21), 16.3 (C-18), 17.1 (C-27), 19.4 (C-19), 20.8 (C-11), 27.6 (C-2), 28.8 (C-24), 30.3 (C-25), 31.3 (C-23), 31.4 (C-8), 31.8 (C-15), 32.0 (C-7), 36.8 (C-10), 36.9 (C-1), 37.9 (C-4), 39.7 (C-12), 40.2 (C-13), 41.6 (C-20), 49.9 (C-9), 56.4 (C-14), 61.9 (C-17), 66.8 (C-26), 75.6 (C-3), 80.8 (C-16) 85.3 (C-3′), 89.9 (C-4′), 109.3 (C-22), 113.9 (C-2′), 114.7 (C-4″), 122.8 (C-6), 123.0 (C-1″), 123.5 (C-4‴), 128.3 (2C), 128.4, 129.3 (2C), 131.5 (2C), 131.9 (C-2″,6″,3″,5″,2‴,6‴,3‴,5‴), 139.4 (C-5), 142.1 (C-1‴), 163.4 (C-1′). IR (KBr, ν, cm−1): 3325 (NH), 2192 (C≡C), 1715 (C=O), 1522 (C=N), 1068, 1051, 1023, 1008 (C-O-C), 1602, 1502, 838, 799, 787, 753, 688 (C=C). HR-MS, m/z (Irel., %): 660 (10), 397 (31), 396 (23), 324 (36), 282 (45), 264 (47), 193 (35), 139 (100), 115 (32), 93 (26), 91 (36), 77 (28). Calcd. C43H52N2O4, m/z [M]+ 660.3922. Found, 660.3918. X-ray structural analysis of compound 20: C43H52N2O4, M 660.87, monoclinic, C2, a 31.72(1), b 7.361(2), c 18.279(5) Å, β 118.52(1)º, V 3750(2) Å3, Z 4, Dcalcd 1.171 g·cm−3, μ(Mo-Kα) 0.074 mm−1, F(000) 1424, (θ 2.27–25.53°, completeness 100%), yellow, (0.26 × 0.21 × 0.04) mm3, transmission 0.7563–0.8596. The intensities of 6672 independent reflections were measured (Rint 0.1263), 446 parameters, 41 restraints, R1 0.0554 (for 3766 observed I > 2σ(I)), wR2 = 0.1614 (all data), GOOF 1.024, largest diff. peak and hole 0.345 and -0.176 e.A−3. Crystallographic data for structure 20 have been deposited at the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC 2077785.
Compound 21. (4S,5′R,6aR,6bS,8aS,8bR,9S,10R,11aS,12aS,12bS)-5′,6a,8a,9-Tetra- methyl-1,3,3′,4,4′,5,5′,6,6a,6b,6′,7,8,8a,8b,9,11a,12,12a,12b-icosahydrospiro(naphtho[2′,1′: 4,5]indeno[2,1-b]furan-10,2′-pyran)-4-yl 1,3-diphenyl-1H-pyrazole-5-carboxylate [(22R,25R)-spirost-5-en-3β-yl 1,3-diphenyl-1H-pyrazole-5-carboxylate] (21). Yield 7% (c). Yellow needles. M.p. 96 °C (decomp.) (petroleum ether–ether, 20:1). [α]D24–76.1 (c 0.2, CHCl3). 1H NMR (600 MHz, CDCl3, δ, ppm): 0.77 (3H, s, H-19), 0.78 (3H, d, J = 6.9 Hz, H-27), 0.97 (3H, d, J = 6.9 Hz, H-21), 1.03 (3H, s, H-18), 1.06–1.33, 1.45–1.69, 1.73–1.80, 1.84–1.89, 1.96–2.02 (21H, m, H-1,1,2,2,4,4,7,7,8,9,11,11,12,12,14,15,15,20,24,24,25), 2.27 (1H, t, J = 12.1 Hz, H-23), 2.33 (1H, dt, J = 12.1, 3.4 Hz, H-23), 3.38 (1H, t, J = 10.9 Hz, H-26), 3.48 (1H, dm, J = 10.9 Hz, H-26), 4.41 (1H, dd, J = 15.2, 7.7 Hz, H-16), 4.72 (1H, m, H-3), 5.37 (1H, d, J = 4.7 Hz, H-6). 7.34 (1H, s, H-4′), 7.36, 7.43, 7.47–7.49, 7.88 (10H, m, H-2″,3″,4″,5″,6″,2‴,3‴,4‴,5‴,6‴). 13C NMR (126 MHz, CDCl3, δ, ppm): 14.5 (C-21), 16.2 (C-18), 17.1 (C-27), 19.2 (C-19), 20.8 (C-11), 27.5 (C-2), 28.8 (C-24), 30.3 (C-25), 31.3 (C-8), 31.4 (C-23), 31.8 (C-15), 32.0 (C-7), 36.7 (C-10), 36.83 (C-1), 37.8 (C-4), 39.7 (C-12), 40.2 (C-13), 41.6 (C-20), 49.9 (C-9), 56.4 (C-14), 62.1 (C-17), 66.8 (C-26), 75.0 (C-3), 80.8 (C-16), 109.2 (C-22), 109.4 (C-4′), 122.7 (C-6), 125.8 (2C), 126.2 (2C), 128.3, 128.5, 128.6 (2C), 128.7 (2c) (C-2″,3″,4″,5″,6″, 2‴,3‴,4‴,5‴,6‴), 132.2 (C-1″), 135.1 (C-3′), 139.3 (C-1‴), 140.5 (C-5), 151.4 (C-5′), 158.5 (C=O). IR (KBr, ν, cm−1): 1728 (C=O), 1543 (C=N pyrazole), 982, 1072, 1053, 1009 (C-O-C), 1599, 1500, 833, 796, 762, 692 (C=C). HR-MS, m/z (Irel., %): 660 (2), 397 (33), 396 (100.00), 283 (26), 282 (98), 265 (87), 264 (55), 247 (25), 139 (95), 69 (29). Calcd. C43H52N2O4, m/z [M]+ 660.3922. Found 660.3897. X-ray structural analysis of compound 21: C43H52N2O4, M 660.87, monoclinic, P21, a 14.2635(6), b 9.7194(4), c 26.781(1), Å, β 95.085(2)°, V 3698.1(3) Å3, Z 4, Dcalcd 1.187.0g·cm−3, μ(Mo-Kα) 0.075 mm−1, F(000) 1424, (θ 0.76–25.06°, completeness 99.9%), colorless, (1.0 × 0.14 × 0.03) mm3, transmission 0. 0.7472–0.8620. The intensities of 12972 independent reflections were measured (Rint 0.0587), 891 parameters, 4 restraints, R1 0.0499 (for 8000 observed I > 2σ(I)), wR2 = 0.1360 (all data), GOOF 1.058, largest diff. peak and hole 0.174 and -0.214 e.A−3. Crystallographic data for structure 21 have been deposited at the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC 2077786.