Investigation of the Optical Properties of a Novel Class of Quinoline Derivatives and Their Random Laser Properties Using ZnO Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Computational Studies of 5d
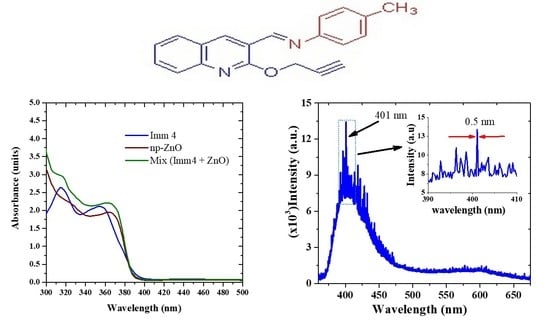
2.3. Experimental Study of Steady-State Optical Properties
2.4. Random Laser Studies
3. Materials and Methods
3.1. Synthesis of Propargylated Quinoline Carbaldehyde, 3
3.2. General Synthetic Procedure for (E)-N-benzylidene-2-(prop-2-yn-1-yloxy)quinolin-3-amine, 5a–h
3.3. Spectral Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sokolov, A.N.; Roberts, M.E.; Bao, Z. Fabrication of low-cost electronic biosensors. Mater. Today 2009, 12, 12–20. [Google Scholar] [CrossRef]
- Someya, T.; Bauer, S.; Kaltenbrunner, M. Imperceptible organic electronics. MRS Bull. 2017, 42, 124–130. [Google Scholar] [CrossRef]
- Abdulrazzaq, O.; Saini, V.; Bourdo, S.; Dervishi, E.; Biris, A.S. Organic Solar Cells: A Review of Materials, Limitations, and Possibilities for Improvement. Part. Sci. Technol. 2013, 31, 427–442. [Google Scholar] [CrossRef]
- Kim, S.-J.; Lee, J.-S. Flexible Organic Transistor Memory Devices. Nano Lett. 2010, 10, 2884–2890. [Google Scholar] [CrossRef]
- Peltomaa, R.; Glahn-Martínez, B.; Benito-Peña, E.; Moreno-Bondi, M.C. Optical Biosensors for Label-Free Detection of Small Molecules. Sensors 2018, 18, 4126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veronese, F.; Morpurgo, M. Bioconjugation in pharmaceutical chemistry. Il Farm. 1999, 54, 497–516. [Google Scholar] [CrossRef]
- Zhu, R.; Ding, J.; Xu, Y.; Yang, J.; Xu, Q.; Pang, H. π-Conjugated Molecule Boosts Metal–Organic Frameworks as Efficient Oxygen Evolution Reaction Catalysts. Small 2018, 14, 1803576. [Google Scholar] [CrossRef]
- Jin, L.; Ma, R.; Liu, H.; Xu, W.; Luo, Z.; Liu, T.; Su, W.; Li, Y.; Lu, R.; Lu, X.; et al. Boosting Highly Efficient Hydrocarbon Solvent-Processed All-Polymer-Based Organic Solar Cells by Modulating Thin-Film Morphology. ACS Appl. Mater. Interfaces 2021, 13, 34301–34307. [Google Scholar] [CrossRef] [PubMed]
- Babel, A.; Jenekhe, S.A. High Electron Mobility in Ladder Polymer Field-Effect Transistors. J. Am. Chem. Soc. 2003, 125, 13656–13657. [Google Scholar] [CrossRef]
- Brown, A.R.; Greenham, N.C.; Gymer, R.W.; Pichler, K.; Bradley, D.D.C.; Friend, R.H.; Burn, P.; Kraft, A.; Holmes, A.B. Conjugated Polymer Light-emitting Diodes. In Intrinsically Conducting Polymers: An Emerging Technology; Springer: Singapore, 1993; pp. 87–106. [Google Scholar]
- Borchert, J.W.; Zschieschang, U.; Letzkus, F.; Giorgio, M.; Weitz, R.T.; Caironi, M.; Burghartz, J.N.; Ludwigs, S.; Klauk, H. Flexible low-voltage high-frequency organic thin-film transistors. Sci. Adv. 2020, 6, eaaz5156. [Google Scholar] [CrossRef]
- Khalid, M.; Lodhi, H.M.; Khan, M.U.; Imran, M. Structural parameter-modulated nonlinear optical amplitude of acceptor–π–D–π–donor-configured pyrene derivatives: A DFT approach. RSC Adv. 2021, 11, 14237–14250. [Google Scholar] [CrossRef]
- Liu, D.; De, J.; Gao, H.; Ma, S.; Ou, Q.; Li, S.; Qin, Z.; Dong, H.; Liao, Q.; Xu, B.; et al. Organic Laser Molecule with High Mobility, High Photoluminescence Quantum Yield, and Deep-Blue Lasing Characteristics. J. Am. Chem. Soc. 2020, 142, 6332–6339. [Google Scholar] [CrossRef] [PubMed]
- Aljaafreh, M.J.; Prasad, S.; AlSalhi, M.S.; AlAhmed, Z.A.; Al-Mogren, M.M. Optically Pumped Intensive Light Amplification from a Blue Oligomer. Polymers 2019, 11, 1534. [Google Scholar] [CrossRef] [Green Version]
- Stefanska, D.; Suski, M.; Furmann, B. Tunable continuous wave single-mode dye laser directly pumped by a diode laser. Laser Phys. Lett. 2017, 14, 045701. [Google Scholar] [CrossRef]
- Brito-Silva, A.M.; Galembeck, A.; Gomes, A.S.L.; Jesus-Silva, A.J.; de Araújo, C.B. Random laser action in dye solutions containing Stöber silica nanoparticles. J. Appl. Phys. 2010, 108, 033508. [Google Scholar] [CrossRef]
- Khalid, M.; Ali, A.; Jawaria, R.; Asghar, M.A.; Asim, S.; Khan, M.U.; Hussain, R.; Rehman, M.F.U.; Ennis, C.J.; Akram, M.S. First principles study of electronic and nonlinear optical properties of A–D–π–A and D–A–D–π–A configured compounds containing novel quinoline–carbazole derivatives. RSC Adv. 2020, 10, 22273–22283. [Google Scholar] [CrossRef]
- Al-Ostoot, F.H.; Salah, S.; Khanum, S.A. Recent investigations into synthesis and pharmacological activities of phenoxy acetamide and its derivatives (chalcone, indole and quinoline) as possible therapeutic candidates. J. Iran. Chem. Soc. 2021, 18, 1–37. [Google Scholar] [CrossRef]
- Thangaraj, M.; Gengan, R.M.; Ranjan, B.; Muthusamy, R. Synthesis, molecular docking, antimicrobial, antioxidant and toxicity assessment of quinoline peptides. J. Photochem. Photobiol. B Biol. 2018, 178, 287–295. [Google Scholar] [CrossRef]
- Yadav, P.; Shah, K. Quinolines, a perpetual, multipurpose scaffold in medicinal chemistry. Bioorgan. Chem. 2021, 109, 104639. [Google Scholar] [CrossRef]
- Khalid, M.; Ali, A.; Asim, S.; Tahir, M.N.; Khan, M.U.; Vieira, L.C.C.; de la Torre, A.F.; Usman, M. Persistent prevalence of supramolecular architectures of novel ultrasonically synthesized hydrazones due to hydrogen bonding [X–H⋯O.; X=N]: Experimental and density functional theory analyses. J. Phys. Chem. Solids 2021, 148, 109679. [Google Scholar] [CrossRef]
- Khalid, M.; Jawaria, R.; Khan, M.U.; Braga, A.A.C.; Shafiq, Z.; Imran, M.; Zafar, H.M.A.; Irfan, A. An Efficient Synthesis, Spectroscopic Characterization, and Optical Nonlinearity Response of Novel Salicylaldehyde Thiosemicarbazone Derivatives. ACS Omega 2021, 6, 16058–16065. [Google Scholar] [CrossRef]
- Hoffmann, A.; Rohrmüller, M.; Jesser, A.; Vieira, I.D.S.; Schmidt, W.G.; Herres-Pawlis, S. Geometrical and optical benchmarking of copper(II) guanidine-quinoline complexes: Insights from TD-DFT and many-body perturbation theory (part II). J. Comput. Chem. 2014, 35, 2146–2161. [Google Scholar] [CrossRef] [PubMed]
- Catalan, J.; del Valle, J.C. Toward the photostability mechanism of intramolecular hydrogen bond systems. The photophysics of 1′-hydroxy-2′-acetonaphthone. J. Am. Chem. Soc. 1993, 115, 4321–4325. [Google Scholar] [CrossRef]
- Paul, B.K.; Guchhait, N. TD–DFT investigation of the potential energy surface for Excited-State Intramolecular Proton Transfer (ESIPT) reaction of 10-hydroxybenzo[h]quinoline: Topological (AIM) and population (NBO) analysis of the intramolecular hydrogen bonding interaction. J. Lumin. 2011, 131, 1918–1926. [Google Scholar] [CrossRef]
- Aljaafreh, M.J.; Prasad, S.; AlSalhi, M.S.; AlAhmed, Z.A. Ultrafast dynamics of laser from green conjugated-oligomer in solution. Polymer 2019, 169, 106–114. [Google Scholar] [CrossRef]
- Elzupir, A.; Ibnaouf, K.; Idriss, H.; Ibrahem, M.; Prasad, S.; Alrajhi, M.; AlSalhi, M.; Alaamer, A. Synthesis and Characterization of an Efficient New Liquid Laser Dye Material-Chalcone (DMAPPP). Acta Phys. Pol. A 2018, 133, 121–125. [Google Scholar] [CrossRef]
- Karstens, T.; Kobs, K. Rhodamine B and rhodamine 101 as reference substances for fluorescence quantum yield measurements. J. Phys. Chem. 1980, 84, 1871–1872. [Google Scholar] [CrossRef]
- Ibnaouf, K.; Prasad, S.; Al Salhi, M.; Hamdan, A.; Zaman, M.; El Mir, L. Influence of the solvent environments on the spectral features of CdSe quantum dots with and without ZnS shell. J. Lumin. 2014, 149, 369–373. [Google Scholar] [CrossRef]
- Aal, N.A.; Al-Hazmi, F.; Al-Ghamdi, A.A.; A Alghamdi, A.; El-Tantawy, F.; Yakuphanoglu, F. Novel rapid synthesis of zinc oxide nanotubes via hydrothermal technique and antibacterial properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 871–877. [Google Scholar] [CrossRef]
- Aljaafreh, M.; Prasad, S.; AlSalhi, M.; Alhandel, R.; Alsaigh, R. TD-DFT Simulation and Experimental Studies of a Mirrorless Lasing of Poly[(9,9-dioctylfluorenyl-2,7-diyl)-co-(1,4-diphenylene-vinylene-2-methoxy-5-{2-ethylhexyloxy}-benzene)]. Polymer 2021, 13, 1430. [Google Scholar] [CrossRef]
- Prasad, S.; Aljaafreh, M.J.; AlSalhi, M.S. Time-resolved spectroscopy of radiative energy transfer between a conjugated oligomer and polymer in solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 232, 118151. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. Gaussian 16, Revision A. 03, Program Gaussian; Gaussian, Inc.: Wallingford, UK, 2016. [Google Scholar]
- Al-Ahmary, K.M.; Mekheimer, R.A.; Al-Enezi, M.S.; Hamada, N.; Habeeb, M.M. Synthesis, spectrophotometric characterization and DFT computational study of a novel quinoline derivative, 2-amino-4-(2,4,6-trinitrophenylamino)-quinoline-3-carbonitrile. J. Mol. Liq. 2018, 249, 501–510. [Google Scholar] [CrossRef]
- Kumar, G.; Paul, K.; Luxami, V. Deciphering the excited state intramolecular charge-coupled double proton transfer in an asymmetric quinoline–benzimidazole system. New J. Chem. 2020, 44, 12866–12874. [Google Scholar] [CrossRef]












| Compound | Fluorescence Peak (nm) | Absorption Peak (nm) | Stokes Shift (Δλ) | Quantum Yield | |||
|---|---|---|---|---|---|---|---|
| S0–1 | S0–0 | S0–1 | S0–2 | S0–3 | |||
| 5a | 421 | 400 | 351 | 335 | 309 | 70 | 0.21 |
| 5b | 411 | - | 349 | 335 | 308 | 62 | 0.19 |
| 5c | 418 | 403 | 350 | - | 311 | 68 | 0.14 |
| 5d | 420 | 409 | 360 | 344 | 314 | 60 | 0.3 |
| 5e | 420 | 400 | 355 | 345 | 313 | 65 | 0.157 |
| 5f | - | 401 | 350 | - | 315 | 51 | 0.18 |
| 5g | - | 409 | 356 | 344 | 312 | 53 | 0.193 |
| 5h | - | 408 | 351 | 336 | 308 | 57 | 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almansour, A.I.; Arumugam, N.; Prasad, S.; Kumar, R.S.; Alsalhi, M.S.; Alkaltham, M.F.; Al-Tamimi, H.b.A. Investigation of the Optical Properties of a Novel Class of Quinoline Derivatives and Their Random Laser Properties Using ZnO Nanoparticles. Molecules 2022, 27, 145. https://doi.org/10.3390/molecules27010145
Almansour AI, Arumugam N, Prasad S, Kumar RS, Alsalhi MS, Alkaltham MF, Al-Tamimi HbA. Investigation of the Optical Properties of a Novel Class of Quinoline Derivatives and Their Random Laser Properties Using ZnO Nanoparticles. Molecules. 2022; 27(1):145. https://doi.org/10.3390/molecules27010145
Chicago/Turabian StyleAlmansour, Abdulrahman I., Natarajan Arumugam, Saradh Prasad, Raju Suresh Kumar, Mohamad S. Alsalhi, Manal Fahad Alkaltham, and Haya bint Abdulaziz Al-Tamimi. 2022. "Investigation of the Optical Properties of a Novel Class of Quinoline Derivatives and Their Random Laser Properties Using ZnO Nanoparticles" Molecules 27, no. 1: 145. https://doi.org/10.3390/molecules27010145