Effect of Pyrolysis Temperature on the Characterisation of Dissolved Organic Matter from Pyroligneous Acid
Abstract
:1. Introduction
2. Results and Discussion
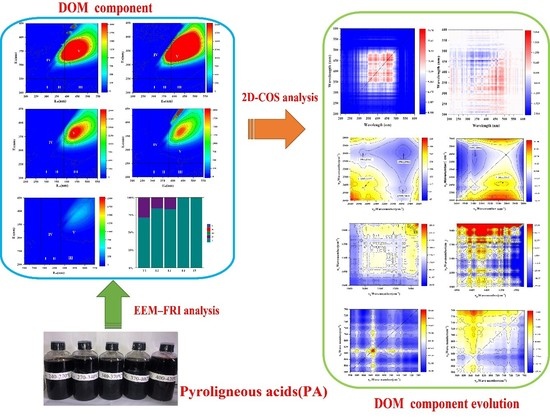
2.1. Difference in DOM of PA at Different Temperature Ranges Characterised via Excitation–Emission Matrix-Fluorescence Regional Integration
2.1.1. Fluorescent Components
2.1.2. Variations in DOM Quality Indices
2.1.3. Variations in DOC Released from PA at Different Temperature Ranges
2.2. Two-Dimensional Correlation Spectroscopic Analysis
2.2.1. Two-Dimensional Correlation Analysis of FTIR
2.2.2. Two-Dimensional Correlation Fluorescence Spectroscopy
2.3. PA Components at Different Temperature Ranges
2.4. Relationship between Chemical Components and Spectral Parameters
3. Materials and Methods
3.1. Pyroligneous Acid Production
3.2. Excitation–Emission Matrix Fluorescence Measurements and Fluorescence Regional Integration Analysis
3.3. Fourier-Transform Infrared Spectroscopy
3.4. Gas Chromatography–Mass Spectroscopy
3.5. Two-Dimensional Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fan, Q.; Li, Y.; Zhao, Y.; Xu, H.; Chen, L.; Hua, D. Anaerobic digestion coupled with three-dimensional iron-carbon electrolysis for enhanced treatment of wood-vinegar wastewater and bacterial structure changes. J. Clean. Prod. 2020, 122095. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.; Wu, S.; Sun, M.; Lyu, J. Multi-purpose production with valorization of wood vinegar and briquette fuels from wood sawdust by hydrothermal process. Fuel 2020, 282, 118775. [Google Scholar] [CrossRef]
- Zhang, F.; Shao, J.; Yang, H.; Guo, D.; Chen, Z.; Zhang, S.; Chen, H. Effects of biomass pyrolysis derived wood vinegar on microbial activity and communities of activated sludge. Bioresour. Technol. 2019, 279, 252–261. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.; Wu, S.; Cao, Z.; Zhang, Y.; Li, H.; Jiang, H.; Lyu, J. Study on an alternative approach for the preparation of wood vinegar from the hydrothermolysis process of cotton stalk. Bioresour. Technol. 2018, 254, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Sun, C.; Hou, X.; Wu, M.; Yao, Y.; Li, F. Pyrolysis of Arundo donax L. to produce pyrolytic vinegar and its effect on the growth of dinoflagellate Karenia brevis. Bioresour. Technol. 2017, 247, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cao, X.; Zhao, L. Comparison of rice husk-and dairy manure-derived bio-chars for simultaneously removing heavy metals from aqueous solutions: Role of mineral components in biochars. Chemosphere 2013, 92, 955–961. [Google Scholar] [CrossRef]
- Li, F.; Zhang, L.; Li, X.; Xie, Y.; Wang, Y.; Wang, J. Biomass co-pyrolysis with calcium dihydrogen phosphate improving carbon fixation of biochar. Trans. Chin. Soc. Agric. Eng. 2016, 32, 201–205. [Google Scholar]
- Qu, X.; Fu, H.; Mao, J.; Ran, Y.; Zhang, D.; Zhu, D. Chemical and structural properties of dissolved black carbon released from biochars. Carbon 2016, 96, 759–767. [Google Scholar] [CrossRef]
- Quan, G.; Fan, Q.; Zimmerman, A.R.; Sun, J.; Cui, L.; Wang, H.; Gao, B.; Yan, J. Effects of laboratory biotic aging on the characteristics of biochar and its water-soluble organic products. J. Hazard. Mater. 2020, 382, 121071. [Google Scholar] [CrossRef]
- Liu, H.; Xu, H.; Wu, Y.; Ai, Z.; Zhang, J.; Liu, G.; Xue, S. Effects of natural vegetation restoration on dissolved organic matter (DOM) biodegradability and its temperature sensitivity. Water Res. 2021, 191, 116792. [Google Scholar] [CrossRef]
- Ren, M.; Horn, H.; Frimmel, F.H. Aggregation behavior of TiO2 nanoparticles in municipal effluent: Influence of ionic strengthen and organic compounds. Water Res. 2017, 123, 678–686. [Google Scholar] [CrossRef]
- Zhang, F.; Li, X.; Duan, L.; Zhang, H.; Gu, W.; Yang, X.; Li, J.; He, S.; Yu, J.; Ren, M. Effect of different DOM components on arsenate complexation in natural water. Environ. Pollut. 2021, 270, 116221. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Yao, Q.; Shao, L.; He, P. Toward understanding the role of individual fluorescent components in DOM-metal binding. J. Hazard. Mater. 2012, 215, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, C.; Zhang, W.; Lin, L.; Wang, L.; Niu, L.; Zhang, H.; Wang, P.; Wang, C. Response of bacterial community in composition and function to the various DOM at river confluences in the urban area. Water Res. 2020, 169, 115293. [Google Scholar] [CrossRef]
- Kamjunke, N.; von Tümpling, W.; Hertkorn, N.; Harir, M.; Schmitt-Kopplin, P.; Norf, H.; Weiterea, M.; Herzsprung, P. A new approach for evaluating transformations of dissolved organic matter (DOM) via high-resolution mass spectrometry and relating it to bacterial activity. Water Res. 2017, 123, 513–523. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, X.; Zhao, M.; Zhao, W.; Liu, J.; Tang, J.; Liao, H.; Chen, Z.; Zhou, S. Hyperthermophilic composting accelerates the humification process of sewage sludge: Molecular characterization of dissolved organic matter using EEM–PARAFAC and two-dimensional correlation spectroscopy. Bioresour. Technol. 2019, 274, 198–206. [Google Scholar] [CrossRef]
- Hur, J.; Lee, B.M. Characterization of binding site heterogeneity for copper within dissolved organic matter fractions using two dimensional correlation fluorescence spectroscopy. Chemosphere 2011, 83, 1603–1611. [Google Scholar] [CrossRef]
- Lee, Y.K.; Hong, S.; Hur, J. Copper-binding properties of microplastic-derived dissolved organic matter revealed by fluorescence spectroscopy and two-dimensional correlation spectroscopy. Water Res. 2021, 190, 116775. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation—Emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Song, F.; Wu, F.; Feng, W.; Liu, S.; He, J.; Li, T.; Zhang, J.; Wu, A.; Amarasiriwardena, D.; Xing, B.; et al. Depth-dependent variations of dissolved organic matter composition and humification in a plateau lake using fluorescence spectroscopy. Chemosphere 2019, 225, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Z.; Zhang, L.; Li, Q.; Li, C.; Chen, G.; Zhang, S.; Liu, Q.; Hu, X. Evolution of the functionalities and structures of biochar in pyrolysis of poplar in a wide temperature range. Bioresour. Technol. 2020, 304, 123002. [Google Scholar] [CrossRef]
- Jamieson, T.; Sager, E.; Guéguen, C. Characterization of biochar-derived dissolved organic matter using UV–Visible absorption and excitation–emission fluorescence spectroscopies. Chemosphere 2014, 103, 197–204. [Google Scholar] [CrossRef]
- Sun, H.; Feng, Y.; Xue, L.; Mandal, S.; Wang, H.; Shi, W.; Yang, L. Responses of ammonia volatilization from rice paddy soil to application of wood vinegar alone or combined with biochar. Chemosphere 2020, 242, 125247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Liu, B.; Liu, Q.; Zheng, H.; You, X.; Luo, X.; Li, F. Comparative study of individual and co-application of biochar and wood vinegar on blueberry fruit yield and nutritional quality. Chemosphere 2020, 246, 125699. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Ngo, H.H.; Guo, W.; Xu, W.; Du, B.; Khan, M.S.; Wei, Q. Biosorption performance evaluation of heavy metal onto aerobic granular sludge-derived biochar in the presence of effluent organic matter via batch and fluorescence approaches. Bioresour. Technol. 2018, 249, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Uchimiya, M.; Ohno, T.; He, Z. Pyrolysis temperature-dependent release of dissolved organic carbon from plant, manure, and biorefinery wastes. J. Anal. Appl. Pyrol. 2013, 104, 84–94. [Google Scholar] [CrossRef]
- Wu, H.; Qi, Y.; Dong, L.; Zhao, X.; Liu, H. Revealing the impact of pyrolysis temperature on dissolved organic matter released from the biochar prepared from Typha orientalis. Chemosphere 2019, 228, 264–270. [Google Scholar] [CrossRef]
- Wei, J.; Tu, C.; Yuan, G.; Bi, D.; Wang, H.; Zhang, L.; Theng, B.K. Pyrolysis temperature-dependent changes in the characteristics of biochar-borne dissolved organic matter and its copper binding properties. Bull. Environ. Contam. Toxicol. 2019, 103, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, L.; Li, Q.; Zhao, Y.; Gao, M.; She, Z.; Jin, C. Three-dimensional fluorescence excitation–emission matrix (EEM) spectroscopy with regional integration analysis for assessing waste sludge hydrolysis at different pretreated temperatures. Environ. Sci. Pollut. Res. 2016, 23, 24061–24067. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Munroe, P.; Joseph, S.; Henderson, R.; Ziolkowski, A. Water extractable organic carbon in untreated and chemical treated biochars. Chemosphere 2012, 87, 151–157. [Google Scholar] [CrossRef]
- Speratti, A.B.; Johnson, M.S.; Sousa, H.M.; Dalmagro, H.J.; Couto, E.G. Biochar feedstock and pyrolysis temperature effects on leachate: DOC characteristics and nitrate losses from a Brazilian Cerrado Arenosol mixed with agricultural waste biochars. J. Environ. Manag. 2018, 211, 256–268. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R.; Harris, W. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- Yang, L.; Chang, S.; Shin, H.; Hur, J. Tracking the evolution of stream DOM source during storm events using end member mixing analysis based on DOM quality. J. Hydrol. 2015, 523, 333–341. [Google Scholar] [CrossRef]
- Tang, J.; Li, X.; Luo, Y.; Li, G.; Khan, S. Spectroscopic characterization of dissolved organic matter derived from different biochars and their polycylic aromatic hydro-carbons (PAHs) binding affinity. Chemosphere 2016, 152, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, C.; Ong, H.; Show, P.; Hsieh, T. Torrefaction, pyrolysis and two-stage thermodegradation of hemicellulose, cellulose and lignin. Fuel 2019, 258, 116168. [Google Scholar] [CrossRef]
- Liu, W.; Li, W.; Jiang, H.; Yu, H. Fates of chemical elements in biomass during its pyrolysis. Chem. Rev. 2017, 117, 6367–6398. [Google Scholar] [CrossRef]
- Yang, H.; Li, S.; Liu, B.; Chen, Y.; Xiao, J.; Dong, Z.; Gong, M.; Chen, H. Hemicellulose pyrolysis mechanism based on functional group evolutions by two-dimensional perturbation correlation infrared spectroscopy. Fuel 2020, 267, 117302. [Google Scholar] [CrossRef]
- Leng, E.; Zhang, Y.; Peng, Y.; Gong, X.; Mao, M.; Li, X.; Yu, Y. In situ structural changes of crystalline and amorphous cellulose during slow pyrolysis at low temperatures. Fuel 2018, 216, 313–321. [Google Scholar] [CrossRef]
- Lv, B.; Xing, M.; Yang, J.; Qi, W.; Lu, Y. Chemical and spectroscopic characterization of water extractable organic matter during vermicomposting of cattle dung. Bioresour. Technol. 2013, 132, 320–326. [Google Scholar] [CrossRef]
- Yuan, Y.; Tao, Y.; Zhou, S.G.; Yuan, T.; Lu, Q.; He, J. Electron transfer capacity as a rapid and simple maturity index for compost. Bioresour. Technol. 2012, 116, 428–434. [Google Scholar] [CrossRef]
- Li, X.; Xing, M.; Yang, J.; Huang, Z. Compositional and functional features of humic acid-like fractions from vermicomposting of sewage sludge and cow dung. J. Hazard. Mater. 2012, 185, 740–748. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, S.; Hou, B.; Zheng, H.; Deng, W.; Liu, D.; Tang, W. Study on the preparation of wood vinegar from biomass residues by carbonization process. Bioresour Technol. 2015, 179, 98–103. [Google Scholar] [CrossRef]
- Gui, X.; Liu, C.; Li, F.; Wang, J. Effect of pyrolysis temperature on the composition of DOM in manure-derived biochar. Ecotoxicol. Environ. Saf. 2020, 197, 110597. [Google Scholar] [CrossRef]
- Wei, J.; Tu, C.; Yuan, G.; Zhou, Y.; Wang, H.; Lu, J. Limited Cu (II) binding to biochar DOM: Evidence from C K-edge NEXAFS and EEM-PARAFAC combined with two-dimensional correlation analysis. Sci. Total Environ. 2020, 701, 134919. [Google Scholar] [CrossRef]
- He, X.; Xi, B.; Wei, Z.; Guo, X.; Li, M.; An, D.; Liu, H. Spectroscopic characterization of water extractable organic matter during composting of municipal solid waste. Chemosphere 2011, 82, 541–548. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Y.; Liu, L.; He, Z.; Giesy, J.P.; Bai, Y.; Sun, F.; Wu, F. Cation-induced coagulation of aquatic plant-derived dissolved organic matter: Investigation by EEM-PARAFAC and FT-IR spectroscopy. Environ. Pollut. 2018, 234, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Baken, S.; Degryse, F.; Verheyen, L.; Merckx, R.; Smolders, E. Metal complexation properties of freshwater dissolved organic matter are explained by its aromaticity and by anthropogenic ligands. Environ. Sci. Technol. 2011, 45, 2584–2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Zhao, Y.; Qi, H.; Zhao, X.; Yang, T.; Du, Y.; Zhang, H.; Wei, Z. Identifying the key factors that affect the formation of humic substance during different materials composting. Bioresour. Technol. 2017, 244, 1193–1196. [Google Scholar] [CrossRef]
- Lu, X.; Jiang, J.; He, J.; Sun, K.; Sun, Y. Effect of pyrolysis temperature on the characteristics of wood vinegar derived from Chinese fir waste: A comprehensive study on its growth regulation performance and mechanism. ACS Omega 2019, 4, 19054–19062. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Tang, Z.; Xu, Y.; Shen, Q. Multiple fluorescence labeling and two dimensional FTIR–13C NMR heterospectral correlation spectroscopy to characterize extracellular polymeric substances in biofilms produced during composting. Environ. Sci. Technol. 2011, 45, 9224–9231. [Google Scholar] [CrossRef]
- Huang, M.; Li, Z.; Luo, N.; Yang, R.; Wen, J.; Huang, B.; Zeng, G. Application potential of biochar in environment: Insight from degradation of biochar-derived DOM and complexation of DOM with heavy metals. Sci. Total. Environ. 2019, 646, 220–228. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Q.; Hu, W.; Qin, J.; Zheng, Y.; Wang, J.; Wang, Q.; Xu, Y.; Guo, G.; Hu, S. Effects of plastic mulch film residues on soil-microbe-plant systems under different soil pH conditions. Chemosphere 2020, 128901. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, Q.; Guo, G.; Qin, J.; Luo, J.; Zhu, Z.; Hong, Y.; Xu, Y.; Hu, S.; Hu, W. Reducing bioavailability of heavy metals in contaminated soil and uptake by maize using organic-inorganic mixed fertilizer. Chemosphere 2020, 261, 128122. [Google Scholar] [CrossRef]
- Chen, W.; Habibul, N.; Liu, X.; Sheng, G.; Yu, H. FTIR and synchronous fluorescence heterospectral two-dimensional correlation analyses on the binding characteristics of copper onto dissolved organic matter. Environ. Sci. Technol. 2015, 49, 2052–2058. [Google Scholar] [CrossRef]
- Noda, I.; Ozaki, Y. Two-Dimensional Correlation Spectroscopy Applications in Vibrational and Optical Spectroscopy; John Wiley & Sons, Ltd.: London, UK, 2004. [Google Scholar]





| Temperature | DOC (mg/L) | Fn (335) | FI | BIX | HIX |
|---|---|---|---|---|---|
| T1 | 699.30 ± 0.91 b | 4219.92 ± 131.08 b | 1.91 ± 0.01 b | 1.13 ± 0.00 b | 1.66 ± 0.02 b |
| T2 | 855.46 ± 4.28 a | 6549.58 ± 252.42 a | 2.24 ± 0.04 a | 1.38 ± 0.03 a | 8.20 ± 4.81 a |
| T3 | 1030.33 ± 4.04 c | 1931.14 ± 95.88 c | 1.66 ± 0.02 c | 0.81 ± 0.03 c | 0.06 ± 0.03 c |
| T4 | 1245.33 ± 17.00 d | 1203.14 ± 785.21 d | 1.96 ± 0.04 b | 0.64 ± 0.55 c | 0.16 ± 0.18b c |
| T5 | 1532.66 ± 11.59 e | 279.45 ± 34.52 e | 1.90 ± 0.04 b | 0.08 ± 0.13 d | 0.40 ± 0.02b c |
| Position (cm−1) | 984 | 1185 | 1295 | 1601 | 1684 |
|---|---|---|---|---|---|
| 984 | + | +(−) | +(−) | +(−) | +(−) |
| 1185 | + | +(+) | +(−) | +(+) | |
| 1295 | + | +(+) | +(+) | ||
| 1601 | + | +(+) | |||
| 1684 | + |
| Position (cm−1) | 320 | 360 | 380 | 450 |
|---|---|---|---|---|
| 320 | + | +(−) | +(−) | +(−) |
| 360 | + | +(+) | +(−) | |
| 380 | + | +(−) | ||
| 450 | + |
| Index | Definition |
|---|---|
| Fn (355) | Fluorescence signal intensity at Ex=355 nm, Em=450 nm |
| HIX (Humification index) | Region integral ratio between Em=435–480 nm and Em=300–345 nm at Ex=245 nm. |
| FI (Fluorescence index) | Ex=370 nm, ratio of between Em=470 nm and Em=520 nm. |
| BIX (Autochthonous index) | Ratio of fluorescence intensity at Em=380–430 nm at Ex=310 nm |
| Ψ (ν1, ν2) | Φ (ν1, ν2) | Interpretation |
|---|---|---|
| + | Intensity of ν1 and ν2 are changing in the same direction | |
| − | Intensity of ν1 and ν2 are changing in the opposite direction | |
| + | + | Change at ν1 is occurring predominantly before that at ν2 |
| − | + | Change at ν1 is occurring predominantly after that at ν2 |
| − | − | Change at ν1 is occurring predominantly before that at ν2 |
| + | − | Change at ν1 is occurring predominantly after that at ν2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, G.; Wang, Q.; Huang, Q.; Fu, Q.; Liu, Y.; Wang, J.; Hu, S.; Mašek, O.; Wang, L.; Zhang, J. Effect of Pyrolysis Temperature on the Characterisation of Dissolved Organic Matter from Pyroligneous Acid. Molecules 2021, 26, 3416. https://doi.org/10.3390/molecules26113416
Guo G, Wang Q, Huang Q, Fu Q, Liu Y, Wang J, Hu S, Mašek O, Wang L, Zhang J. Effect of Pyrolysis Temperature on the Characterisation of Dissolved Organic Matter from Pyroligneous Acid. Molecules. 2021; 26(11):3416. https://doi.org/10.3390/molecules26113416
Chicago/Turabian StyleGuo, Genmao, Qingqing Wang, Qing Huang, Qionglin Fu, Yin Liu, Junfeng Wang, Shan Hu, Ondřej Mašek, Luya Wang, and Ju Zhang. 2021. "Effect of Pyrolysis Temperature on the Characterisation of Dissolved Organic Matter from Pyroligneous Acid" Molecules 26, no. 11: 3416. https://doi.org/10.3390/molecules26113416