Viburnum stellato-tomentosum Extract Suppresses Obesity and Hyperglycemia through Regulation of Lipid Metabolism in High-Fat Diet-Fed Mice
Abstract
:1. Introduction
2. Results
2.1. HPLC Analysis of VS Extract
2.2. Effect of VS Extract on Body and Organ Weights
2.3. Effect of VS Extract on Metabolic Parameters and Glucose Homeostasis
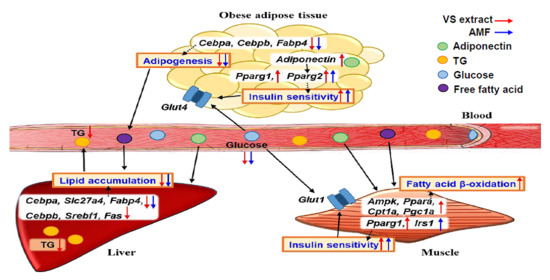
2.4. Effect of VS Extract on Adipocyte Hypertrophy and Insulin Sensitivity in WAT
2.5. Effect of VS Extract on HFD-induced Hepatic Steatosis
2.6. Effects of VS Extract on Fatty Acid β-oxidation and Insulin Sensitivity in the Skeletal Muscle
2.7. AMF Regulates Lipogenesis and Insulin Signaling in HFD-Fed Mice
3. Discussion
4. Materials and Methods
4.1. Preparation of VS and HPLC Analysis
4.2. Animals and Diets
4.2.1. Experiment 1
4.2.2. Experiment 2
4.3. Measurements of Body and Organ Weights and Biochemical Parameters
4.4. Oral Glucose Tolerance Test (OGTT)
4.5. Histological Analysis of WAT and Liver
4.6. Measurement of Hepatic Lipid Contents
4.7. Quantitative Real-Time RT-PCR (qRT-PCR)
4.8. Western Blot Analyses
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| AMF | amentoflavone |
| AMPK | AMP-activated protein kinase |
| C/EBP | CCAAT/enhancer-binding protein |
| CPT1α | carnitine palmitoyltransferase 1α |
| FABP | fatty acid binding protein |
| FAS | fatty acid synthase |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GLUT | glucose transporter |
| HbA1c | glycated hemoglobin |
| HOMA-IR | homeostasis model assessment of insulin resistance |
| IR | insulin resistance |
| IRS | insulin receptor substrate |
| NEFAs | non-esterified fatty acids |
| OGTT | oral glucose tolerance test |
| PGC1α | peroxisome proliferator activated receptor gamma coactivator 1α |
| PPAR | peroxisome proliferator-activated receptor |
| SLC27A4 | solute carrier family 27 member 4 |
| SREBF1 | sterol regulatory element-biding transcription factor 1 |
| T2DM | type 2 diabetes mellitus |
| TNFα | tumor necrosis factor α |
| UCP | uncoupling protein |
| VS | Viburnum stellato-tomentosum |
| WAT | white adipose tissue |
References
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metab. Clin. Exp. 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leal-Díaz, A.M.; Noriega, L.G.; Torre-Villalvazo, I.; Torres, N.; Alemán-Escondrillas, G.; López-Romero, P.; Sánchez-Tapia, M.; Aguilar-López, M.; Furuzawa-Carballeda, J.; Velázquez-Villegas, L.A. Aguamiel concentrate from Agave salmiana and its extracted saponins attenuated obesity and hepatic steatosis and increased Akkermansia muciniphila in C57BL6 mice. Sci. Rep. 2016, 6, 34242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hebbard, L.; George, J. Animal models of nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol Hepatol. 2011, 8, 35. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metab. Clin. Exp. 2019, 92, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Bastard, J.-P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006, 17, 4–12. [Google Scholar]
- Debard, C.; Laville, M.; Berbe, V.; Loizon, E.; Guillet, C.; Morio-Liondore, B.; Boirie, Y.; Vidal, H. Expression of key genes of fatty acid oxidation, including adiponectin receptors, in skeletal muscle of type 2 diabetic patients. Diabetologia 2004, 47, 917–925. [Google Scholar] [CrossRef] [Green Version]
- McGarry, J.D. Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 2002, 51, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Morton, C.V. The Mexican and Central American species of Viburnum. In Systematic plant studies: Mainly tropical America; Contributions from the United States National Herbarium: Washington, DC, USA, 1933; pp. 339–366. [Google Scholar]
- Lobstein, A.; Haan-Archipoff, G.; Englert, J.; Kuhry, J.-G.; Anton, R. Chemotaxonomical investigation in the genus Viburnum. Phytochemistry 1999, 50, 1175–1180. [Google Scholar] [CrossRef]
- Chen, G.; Han, Y.; He, W.; Liang, F. Amentoflavone protects against high fat-induced metabolic dysfunction: Possible role of the regulation of adipogenic differentiation. Int. J. Mol. Med. 2016, 38, 1759–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kintscher, U.; Law, R.E. PPARγ-mediated insulin sensitization: The importance of fat versus muscle. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E287–E291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, H.M.; Holloway, G.P.; Steinberg, G.R. AMPK regulation of fatty acid metabolism and mitochondrial biogenesis: Implications for obesity. Mol. Cell Endocrinol. 2013, 366, 135–151. [Google Scholar] [CrossRef]
- Iwai, K.; Kim, M.-Y.; Onodera, A.; Matsue, H. Physiological effects and active ingredients of Viburnum dilatatum Thunb fruits on oxidative stress. Biofactors 2004, 21, 273–275. [Google Scholar] [CrossRef]
- Iwai, K.; Kim, M.-Y.; Onodera, A.; Matsue, H. α-Glucosidase inhibitory and antihyperglycemic effects of polyphenols in the fruit of Viburnum dilatatum Thunb. J. Agric. Food Chem. 2006, 54, 4588–4592. [Google Scholar] [CrossRef]
- Zaklos-Szyda, M.; Pawlik, N.; Polka, D.; Nowak, A.; Koziolkiewicz, M.; Podsedek, A. Viburnum opulus fruit phenolic compounds as cytoprotective agents able to decrease free fatty acids and glucose uptake by Caco-2 cells. Antioxidants 2019, 8, 262. [Google Scholar] [CrossRef] [Green Version]
- Okabe, T.; Toda, T.; Nukitrangsan, N.; Inafuku, M.; Iwasaki, H.; Oku, H. Peucedanum japonicum Thunb inhibits high-fat diet induced obesity in mice. Phytother Res. 2011, 25, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.; Newgard, C.B.; et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.J.; Song, K.-H.; Koh, E.H.; Won, J.C.; Kim, H.S.; Park, H.-S.; Kim, M.-S.; Kim, S.-W.; Lee, K.-U.; Park, J.-Y. α-Lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem. Biophys. Res. Commun. 2005, 332, 885–891. [Google Scholar] [CrossRef]
- Qin, B.; Nagasaki, M.; Ren, M.; Bajotto, G.; Oshida, Y.; Sato, Y. Cinnamon extract (traditional herb) potentiates in vivo insulin-regulated glucose utilization via enhancing insulin signaling in rats. Diabetes Res. Clin. Pract. 2003, 62, 139–148. [Google Scholar] [CrossRef]
- Dos Santos, J.M.; Benite-Ribeiro, S.A.; Queiroz, G.; Duarte, J.A. The effect of age on glucose uptake and GLUT1 and GLUT4 expression in rat skeletal muscle. Cell Biochem Funct. 2012, 30, 191–197. [Google Scholar] [CrossRef]
- Chadt, A.; Al-Hasani, H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflugers Arch.-Eur J. Physiol. 2020, 472, 1273–1298. [Google Scholar] [CrossRef]
- Long, Y.C.; Cheng, Z.; Copps, K.D.; White, M.F. Insulin receptor substrates Irs1 and Irs2 coordinate skeletal muscle growth and metabolism via the Akt and AMPK pathways. Mol. Cell Biol. 2011, 31, 430–441. [Google Scholar] [CrossRef] [Green Version]
- Thirone, A.C.; Huang, C.; Klip, A. Tissue-specific roles of IRS proteins in insulin signaling and glucose transport. Trends Endocrinol Metab. 2006, 17, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, M.; Vinayagamoorthi, R.; Suyambunathan, V.A.; Bobby, Z.; Selvaraj, N. Bitter gourd (Momordica charantia) improves insulin sensitivity by increasing skeletal muscle insulin-stimulated IRS-1 tyrosine phosphorylation in high-fat-fed rats. Br. J. Nutr. 2008, 99, 806–812. [Google Scholar] [CrossRef] [Green Version]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A. Obesity, metabolic syndrome, and type 2 diabetes: Inflammatory basis of glucose metabolic disorders. Nutr. Rev. 2007, 65, 152–156. [Google Scholar] [CrossRef]
- Breneman, C.B.; Tucker, L. Dietary fibre consumption and insulin resistance–the role of body fat and physical activity. Br. J. Nutr. 2013, 110, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Okigawa, M.; Hwa, C.W.; Kawano, N.; Rahman, W. Biflavones in Selaginella species. Phytochemistry 1971, 10, 3286–3287. [Google Scholar] [CrossRef]
- Lin, L.-C.; Kuo, Y.-C.; Chou, C.-J. Cytotoxic biflavonoids from Selaginella delicatula. J. Nat. Prod. 2000, 63, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.-C.; But, P.P.-H.; Ooi, V.E.-C.; He, Y.-H.; Lee, S.H.-S.; Lee, S.-F.; Lin, R.-C. Antiviral amentoflavone from Selaginella sinensis. Biol. Pharm. Bull. 2001, 24, 311–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Parameters | ND | HFD | HFD+VS |
|---|---|---|---|
| Food intake (g/day) | 2.5 ± 0.0 | 2.5 ± 0.1 | 2.4 ± 0.2 |
| Energy intake (kcal/day) | 9.8 ± 0.1 | 13.2 ± 0.6 # | 12.5 ± 0.9 |
| Liver (g/kg body weight) | 29.5 ± 0.5 | 37.8 ± 3.3 # | 26.5 ± 1.7 * |
| Pancreas (g/kg body weight) | 4.1 ± 0.2 | 3.9 ± 0.2 | 3.7 ± 0.2 |
| Muscle (g/kg body weight) | 10.9 ± 0.5 | 9.2 ± 0.9 | 9.2 ± 0.7 |
| WAT (g/kg body weight) | |||
| Retroperitoneal AT | 3.0 ± 0.3 | 26.7 ± 0.4 # | 25.0 ± 0.6 * |
| Gonadal AT | 13.8 ± 0.9 | 61.4 ± 3.0 # | 50.4 ± 3.6 * |
| Inguinal AT | 8.2 ± 0.9 | 54.6 ± 2.2 # | 42.3 ± 3.8 ** |
| Total WAT | 25.0 ± 1.9 | 138.9 ± 7.5 # | 114.3 ± 4.7 * |
| Parameters | ND | HFD | HFD+VS |
|---|---|---|---|
| Glucose (mg/dL) | 76.9 ± 3.6 | 124.3 ± 3.8 # | 110.2 ± 5.7 * |
| HbA1c (%) | 4.8 ± 0.1 | 5.2 ± 0.3 | 5.3 ± 0.2 |
| Insulin (ng/mL) | 0.3 ± 0.1 | 2.2 ± 0.2 # | 1.2 ± 0.3 * |
| HOMA-IR | 1.4 ± 0.2 | 20.4 ± 2.7 # | 8.0 ± 1.8 ** |
| TG (mg/dL) | 146.6 ± 4.1 | 155.6 ± 5.9 # | 128.9 ± 8.4 * |
| TC (mg/dL) | 92.9 ± 4.9 | 225.6 ± 8.7 # | 205.5 ± 3.4 * |
| NEFA (mEq/L) | 2.1 ± 0.2 | 2.2 ± 0.2 | 2.1 ± 0.2 |
| AST (IU/L) | 47.9 ± 2.4 | 60.8 ± 2.8 # | 46.8 ± 4.7 * |
| ALT (IU/L) | 12.1 ± 0.7 | 35.9 ± 3.7 # | 18.4 ± 3.2 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.; Lee, H.; Han, J.; Lee, H.; Kattia, R.O.; Nelson, Z.V.; Choi, S.; Kim, S.-Y.; Park, H.-Y.; Jeong, H.G.; et al. Viburnum stellato-tomentosum Extract Suppresses Obesity and Hyperglycemia through Regulation of Lipid Metabolism in High-Fat Diet-Fed Mice. Molecules 2021, 26, 1052. https://doi.org/10.3390/molecules26041052
Cho S, Lee H, Han J, Lee H, Kattia RO, Nelson ZV, Choi S, Kim S-Y, Park H-Y, Jeong HG, et al. Viburnum stellato-tomentosum Extract Suppresses Obesity and Hyperglycemia through Regulation of Lipid Metabolism in High-Fat Diet-Fed Mice. Molecules. 2021; 26(4):1052. https://doi.org/10.3390/molecules26041052
Chicago/Turabian StyleCho, Seona, Hwa Lee, Jisu Han, Haneul Lee, Rosales Ovares Kattia, Zamora Villalobos Nelson, Sangho Choi, Soo-Yong Kim, Ho-Yong Park, Hye Gwang Jeong, and et al. 2021. "Viburnum stellato-tomentosum Extract Suppresses Obesity and Hyperglycemia through Regulation of Lipid Metabolism in High-Fat Diet-Fed Mice" Molecules 26, no. 4: 1052. https://doi.org/10.3390/molecules26041052