Systematic Investigations of Annealing and Functionalization of Carbon Nanotube Yarns
Abstract
:1. Introduction
2. Results and Discussion
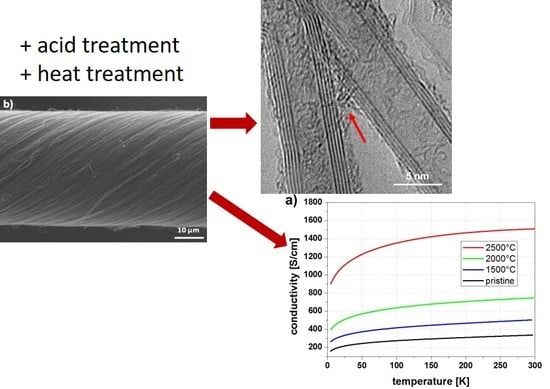
2.1. Annealing
2.2. Acid Treatment
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lekawa-Raus, A.; Patmore, J.; Kurzepa, L.; Bulmer, J.; Koziol, K. Electrical properties of carbon nanotube based fibers and their future use in electrical wiring. Adv. Funct. Mater. 2014, 24, 3661–3682. [Google Scholar] [CrossRef]
- Charlier, J.; Blase, X.; Roche, S. Electronic and transport properties of nanotubes. Rev. Mod. Phys. 2007, 79, 677–732. [Google Scholar] [CrossRef] [Green Version]
- Kim, P.; Shi, L.; Majumdar, A.; McEuen, P.L. Thermal transport measurements of individual multiwalled nanotubes. Phys. Rev. Lett. 2001, 87, 215502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, G.; Çagin, T.; Goddard, W.A. Energetics, structure, mechanical and vibrational properties of single-walled carbon nanotubes. Nanotechnology 1998, 9, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Myung, S. A flexible approach to mobility. Nat. Nanotechnol. 2007, 2, 207–208. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Tu, Y.; Li, Y.; Coulter, J.Y.; Zheng, L.; Zhao, Y.; Jia, Q.; Peterson, D.E.; Zhu, Y.; et al. Strong carbon-nanotube fibers spun from long carbon-nanotube arrays. Small 2007, 3, 244–248. [Google Scholar] [CrossRef]
- Davis, V.A.; Parra-Vasquez, A.N.G.; Green, M.J.; Rai, P.K.; Behabtu, N.; Prieto, V.; Booker, R.D.; Schmidt, J.; Kesselman, E.; Zhou, W.; et al. True solutions of single-walled carbon nanotubes for assembly into macroscopic materials. Nat. Nanotechnol. 2009, 4, 830–834. [Google Scholar] [CrossRef]
- Behabtu, N.; Young, C.C.; Tsentalovich, D.E.; Kleinerman, O.; Wang, X.; Ma, A.W.K.; Bengio, E.A.; Waarbeek, R.F.T.; de Jong, J.J.; Hoogerwerf, R.E.; et al. Strong, Light, Multifunctional Fibers of Carbon Nanotubes with Ultrahigh Conductivity. Science 2013, 339, 182–186. [Google Scholar] [CrossRef] [Green Version]
- Orbaek, A.W.; Aggarwal, N.; Barron, A.R. The development of a ‘process map’ for the growth of carbon nanomaterials from ferrocene by injection CVD. J. Mater. Chem. A 2013, 1, 14122–14132. [Google Scholar] [CrossRef]
- Khanbolouki, P.; Tehrani, M. Viscoelastic behaviour of carbon nanotube yarns and twisted coils. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Pittsburgh, PA, USA, 9–15 November 2018; Volume 12. V012T11A031. [Google Scholar]
- Mclean, B.; Eveleens, C.A.; Mitchell, I.; Webber, G.B.; Page, A.J. Catalytic CVD synthesis of boron nitride and carbon nanomaterials—Synergies between experiment and theory. Phys. Chem. Chem. Phys. 2017, 19, 26466–26494. [Google Scholar] [CrossRef]
- Xu, F.; Sadrzadeh, A.; Xu, Z.; Yakobson, B.I. Can carbon nanotube fibers achieve the ultimate conductivity? Coupled-mode analysis for electron transport through the carbon nanotube contact. J. Appl. Phys. 2013, 114, 063714. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Luo, G.; Wei, F. 99.9% purity multi-walled carbon nanotubes by vacuum high-temperature annealing. Carbon 2003, 41, 2585–2590. [Google Scholar] [CrossRef]
- Yamamoto, G.; Shirasu, K.; Nozaka, Y.; Sato, Y.; Takagi, T.; Hashida, T. Structure–property relationships in thermally-annealed multi-walled carbon nanotubes. Carbon 2014, 66, 219–226. [Google Scholar] [CrossRef]
- Niven, J.F.; Johnson, M.B.; Juckes, S.M.; White, M.A.; Alvarez, N.T.; Shanov, V. Influence of annealing on thermal and electrical properties of carbon nanotube yarns. Carbon 2016, 99, 485–490. [Google Scholar] [CrossRef] [Green Version]
- Pierlot, A.; Woodhead, A.L.; Church, J.S. Thermal annealing effects on multi-walled carbon nanotube yarns probed by Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Hayashi, Y.; Khavrus, V.; Chujo, D.; Inoue, H.; Hada, M.; Leonhardt, A.; Büchner, B.; Hampel, S. Resistance-heating of carbon nanotube yarns in different atmospheres. Carbon 2018, 133, 232–238. [Google Scholar] [CrossRef]
- Gong, T.; Zhang, Y.; Liu, W.; Wei, J.; Wang, K.; Wu, D.; Zhong, M.; Zhong, M. Connection of macro-sized double-walled carbon nanotube strands by current-assisted laser irradiation. J. Laser Appl. 2008, 20, 122–126. [Google Scholar] [CrossRef]
- Skakalova, V.; Kaiser, A.B.; Dettlaff-Weglikowska, U.; Hrnčariková, K.; Roth, S. Effect of chemical treatment on electrical conductivity, infrared absorption, and raman spectra of single-walled carbon nanotubes. J. Phys. Chem. B 2005, 109, 7174–7181. [Google Scholar] [CrossRef]
- Morelos-Gomez, A.; Fujishige, M.; Vega-Díaz, S.M.; Ito, I.; Fukuyo, T.; Cruz-Silva, R.; Tristán-López, F.; Fujisawa, K.; Fujimori, T.; Futamura, R.; et al. High electrical conductivity of double-walled carbon nanotube fibers by hydrogen peroxide treatments. J. Mater. Chem. A 2016, 4, 74–82. [Google Scholar] [CrossRef]
- Liu, P.; Hu, D.C.M.; Tran, T.Q.; Jewell, D. Electrical property enhancement of carbon nanotube fibers from post treatments. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509 (Suppl. C), 384–389. [Google Scholar] [CrossRef]
- Meng, F.; Zhao, J.; Ye, Y.; Zhang, X.; Li, Q. Carbon nanotube fibers for electrochemical applications: Effect of enhanced interfaces by an acid treatment. Nanoscale 2012, 4, 7464–7468. [Google Scholar] [CrossRef]
- Iijima, T.; Oshima, H.; Hayashi, Y.; Suryavanshi, U.B.; Hayashi, A.; Tanemura, M. In-situ observation of carbon nanotube fiber spinning from vertically aligned carbon nanotube forest. Diam. Relat. Mater. 2012, 24, 158–160. [Google Scholar] [CrossRef]
- Endo, M.; Hayashi, T.; Muramatsu, H.; Kim, Y.; Terrones, H.; Terrones, M.; Dresselhaus, M.S. Coalescence of double-walled carbon nanotubes: Formation of novel carbon bicables. Nano Lett. 2004, 4, 1451–1454. [Google Scholar] [CrossRef]
- Andrews, R.; Jacques, D.; Qian, D.; Dickey, E.C. Purification and structural annealing of multiwalled carbon nanotubes at graphitization temperatures. Carbon 2001, 39, 1681–1687. [Google Scholar] [CrossRef]
- Shklovskii, B.I.; Efros, A.L. Variable-range hopping conduction. In Electronic Properties of Doped Semiconductors; Springer: Berlin/Heidelberg, Germany, 1984; pp. 202–227. [Google Scholar]
- Pöhls, J.-H.; Johnson, M.B.; White, M.A.; Malik, R.; Ruff, B.; Jayasinghe, C.; Schulz, M.J.; Shanov, V. Physical properties of carbon nanotube sheets drawn from nanotube arrays. Carbon 2012, 50, 4175–4183. [Google Scholar] [CrossRef]
- Jakubinek, M.; Johnson, M.B.; White, M.A.; Jayasinghe, C.; Li, G.; Cho, W.; Schulz, M.J.; Shanov, V. Thermal and electrical conductivity of array-spun multi-walled carbon nanotube yarns. Carbon 2012, 50, 244–248. [Google Scholar] [CrossRef]
- Jakubinek, M.; White, M.A.; Li, G.; Jayasinghe, C.; Cho, W.; Schulz, M.J.; Schulz, M.J.; Shanov, V. Thermal and electrical conductivity of tall, vertically aligned carbon nanotube arrays. Carbon 2010, 48, 3947–3952. [Google Scholar] [CrossRef]
- Kaiser, A.; Skákalová, V.; Roth, S. Modelling conduction in carbon nanotube networks with different thickness, chemical treatment and irradiation. Phys. E Low Dimens. Syst. Nanostructures 2008, 40, 2311–2318. [Google Scholar] [CrossRef]
- Skákalová, V.; Kaiser, A.B.; Dettlaff-Weglikowska, U.; Hrnčariková, K.; Roth, S. Electronic transport in carbon nanotubes: From individual nanotubes to thin and thick networks. Phys. Rev. B 2006, 74, 085403. [Google Scholar] [CrossRef]
- Khan, Z.; Husain, M.; Perng, T.P.; Salah, N.; Habi, S. Electrical transport via variable range hopping in an individual multi-wall carbon nanotube. J. Phys. Condens. Matter. 2008, 20, 475207. [Google Scholar] [CrossRef]
- Mott, N. Conduction in non-crystalline materials. Philos. Mag. A J. Theor. Exp. Appl. Phys. 1969, 19, 835–852. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, B.; Itkis, M.E.; Haddo, R.C. Nitric acid purification of single-walled carbon nanotubes. J. Phys. Chem. B 2003, 107, 13838–13842. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, H.; Qing, Q.; Yang, Y.; Li, Q.; Liu, Z.; Guo, X.; Du, Z. Effect of chemical oxidation on the structure of single-walled carbon nanotubes. J. Phys. Chem. B 2003, 107, 3712–3718. [Google Scholar] [CrossRef]
- Janas, D.; Vilatela, A.C.; Koziol, K.K.K. Performance of carbon nanotube wires in extreme conditions. Carbon 2013, 62, 438–446. [Google Scholar] [CrossRef]
- Liu, C.H.; Fan, S.S. Effects of chemical modifications on the thermal conductivity of carbon nanotube composites. Appl. Phys. Lett. 2005, 86, 123106. [Google Scholar] [CrossRef]
- Martin, E.; George, C.; Mirabel, P. Densities and surface tensions of H2SO4/HNO3/H2O solutions. Geophys. Res. Lett. 2000, 27, 197–200. [Google Scholar] [CrossRef]
- Nasr-El-Din, H.A.; Al-Othman, A.M.; Taylor, K.C.; Al-Ghamdi, A.H. Surface tension of HCl-based stimulation fluids at high temperatures. J. Pet. Sci. Eng. 2004, 43, 57–73. [Google Scholar] [CrossRef]
- Hawley, G. Hawley’s Condensed Chemical Dictionary, 15th ed.; Wiley: New York, NY, USA, 2007. [Google Scholar]
Sample Availability: Samples of the compounds CNY and different modifications are available from the authors. |










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scholz, M.; Hayashi, Y.; Eckert, V.; Khavrus, V.; Leonhardt, A.; Büchner, B.; Mertig, M.; Hampel, S. Systematic Investigations of Annealing and Functionalization of Carbon Nanotube Yarns. Molecules 2020, 25, 1144. https://doi.org/10.3390/molecules25051144
Scholz M, Hayashi Y, Eckert V, Khavrus V, Leonhardt A, Büchner B, Mertig M, Hampel S. Systematic Investigations of Annealing and Functionalization of Carbon Nanotube Yarns. Molecules. 2020; 25(5):1144. https://doi.org/10.3390/molecules25051144
Chicago/Turabian StyleScholz, Maik, Yasuhiko Hayashi, Victoria Eckert, Vyacheslav Khavrus, Albrecht Leonhardt, Bernd Büchner, Michael Mertig, and Silke Hampel. 2020. "Systematic Investigations of Annealing and Functionalization of Carbon Nanotube Yarns" Molecules 25, no. 5: 1144. https://doi.org/10.3390/molecules25051144