Anti-Inflammatory, Antioxidant, and Hypolipidemic Effects of Mixed Nuts in Atherogenic Diet-Fed Rats
Abstract
:1. Introduction
2. Results
2.1. Effect of Nuts on Food and Water Intake and Body Weight
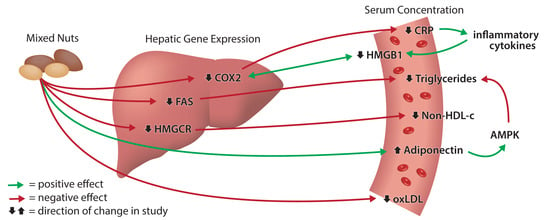
2.2. Effect of Nuts on Serum Lipids and Adiponectin
2.3. Effect of Nuts on Serum CRP, HMGB1, and Oxidative Stress
2.4. Effect of Nuts on Serum Antioxidant Enzyme Activities
2.5. Effect of Nuts on Serum Liver Function Enzyme Activities
2.6. Effect of Nuts on Hepatic Gene Expression
3. Discussion
4. Materials and Methods
4.1. Animals and Diets
4.2. Serum Lipids and Oxidized LDL
4.3. Serum Adiponectin
4.4. Serum C-Reactive Protein
4.5. Serum HMGB1 Activity
4.6. Serum Thiobarbituric Acid Reactive Substances
4.7. Serum Antioxidant Enzyme Activities
4.8. Serum Liver Function Enzyme Activities
4.9. Hepatic Gene Expression
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kochanek, K.D.; Murphy, S.; Xu, J.; Arias, E. Mortality in the United States, 2016. NCHS Data Brief. 2017, 1–8. [Google Scholar]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Relja, A.; Miljković, A.; Gelemanović, A.; Bošković, M.; Hayward, C.; Polašek, O.; Kolčić, I. Nut consumption and cardiovascular risk factors: A cross-sectional study in a mediterranean population. Nutrients 2017, 9, 1296. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Micha, R.; Khatibzadeh, S.; Mozaffarian, D. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Ibarrola-Jurado, N.; Bulló, M.; Guasch-Ferré, M.; Ros, E.; Martínez-González, M.A.; Corella, D.; Fiol, M.; Wärnberg, J.; Estruch, R.; Román, P.; et al. Cross-sectional assessment of nut consumption and obesity, metabolic syndrome and other cardiometabolic risk factors: The predimed study. PLoS ONE 2013, 8, e57367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ros, E.; Mataix, J. Fatty acid composition of nuts – implications for cardiovascular health. Br. J. Nutr. 2006, 96, S29–35. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, R.L.; Etherton, T.D.; Pearson, T.A.; Harrison, E.H.; Kris-Etherton, P.M. Low fat and high monounsaturated fat diets decrease human low density lipoprotein oxidative susceptibility in vitro. J. Nutr. 2001, 131, 1758–1763. [Google Scholar] [CrossRef] [PubMed]
- Lunn, J.; Buttriss, J.L. Carbohydrates and dietary fibre. Nutr. Bull. 2007, 32, 21–64. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Keogh, J.; Clifton, P. Benefits of nut consumption on insulin resistance and cardiovascular risk factors: multiple potential mechanisms of actions. Nutrients 2017, 9, 1271. [Google Scholar] [CrossRef] [PubMed]
- Ward, M. Homocysteine, folate, and cardiovascular disease. Int. J. Vitam. Nutr. Res. 2001, 71, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Pisu, E. Role of dietary magnesium in cardiovascular disease prevention, insulin sensitivity and diabetes. Curr. Opin. Lipidol. 2008, 19, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Zhao, G.; Binkoski, A.E.; Coval, S.M.; Etherton, T.D. The effects of nuts on coronary heart disease risk. Nutr. Rev. 2009, 59, 103–111. [Google Scholar] [CrossRef]
- Bansode, R.R.; Randolph, P.; Ahmedna, M.; Hurley, S.; Hanner, T.; Baxter, S.A.S.; Johnston, T.A.; Su, M.; Holmes, B.M.; Yu, J.; et al. Bioavailability of polyphenols from peanut skin extract associated with plasma lipid lowering function. Food Chem. 2014, 148, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alonso, P.; Salas-Salvadó, J.; Baldrich-Mora, M.; Juanola-Falgarona, M.; Bulló, M. Beneficial effect of pistachio consumption on glucose metabolism, insulin resistance, inflammation, and related metabolic risk markers: A randomized clinical trial. Diabetes Care 2014, 37, 3098–3105. [Google Scholar] [CrossRef] [PubMed]
- Kasliwal, R.R.; Bansal, M.; Mehrotra, R.; Yeptho, K.P.; Trehan, N. Effect of pistachio nut consumption on endothelial function and arterial stiffness. Nutrition 2015, 31, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Misra, A.; Pandey, R.M.; Bhatt, S.P.; Saluja, S. Effects of pistachio nuts on body composition, metabolic, inflammatory and oxidative stress parameters in Asian Indians with metabolic syndrome: A 24-wk, randomized control trial. Nutrition 2014, 30, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.W.C.; West, S.G.; Augustin, L.S.; Esfahani, A.; Vidgen, E.; Bashyam, B.; Sauder, K.A.; Campbell, J.; Chiavaroli, L.; Jenkins, A.L.; et al. Acute effects of pistachio consumption on glucose and insulin, satiety hormones and endothelial function in the metabolic syndrome. Eur. J. Clin. Nutr. 2014, 68, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Sari, I.; Baltaci, Y.; Bagci, C.; Davutoglu, V.; Erel, O.; Celik, H.; Ozer, O.; Aksoy, N.; Aksoy, M. Effect of pistachio diet on lipid parameters, endothelial function, inflammation, and oxidative status: A prospective study. Nutrition 2010, 26, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Covas, M.I.; Fiol, M.; Gómez-Gracia, E.; López-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-style diet on cardiovascular risk factors. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Y.; Brennan, A.M.; Wedick, N.M.; Mantzoros, C.; Rifai, N.; Hu, F.B. Regular consumption of nuts is associated with a lower risk of cardiovascular disease in women with type 2 diabetes. J. Nutr. 2009, 139, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.G.M.; Gomes, A.C.; Naves, M.M.V.; Mota, J.F. Nuts and legume seeds for cardiovascular risk reduction: Scientific evidence and mechanisms of action. Nutr. Rev. 2015, 73, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Malik, V.S.; Keum, N.; Hu, F.B.; Giovannucci, E.L.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S.; Bao, Y. Associations between nut consumption and inflammatory biomarkers. Am. J. Clin. Nutr. 2016, 104, 722–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceriello, A. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? the common soil hypothesis revisited. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Zare Javid, A.; Hormoznejad, R.; Yousefimanesh, H.A.; Zakerkish, M.; Haghighi-zadeh, M.H.; Dehghan, P.; Ravanbakhsh, M. The impact of resveratrol supplementation on blood glucose, insulin, insulin resistance, triglyceride, and periodontal markers in type 2 diabetic patients with chronic periodontitis: Resveratrol in diabetic patients with periodontitis. Phytother. Res. 2017, 31, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Roasted Nuts and Peanut Butter Manufacturing: 2002. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwiLv_CMzvPeAhUHfH0KHRijDgIQFjAAegQIABAC&url=https%3A%2F%2Fwww.census.gov%2Fprod%2Fec02%2Fec0231i311911t.pdf&usg=AOvVaw0a4cAp5xQ8Ge1b4GDYeaJR (accessed on 25 November 2018).
- Lee, Y.J.; Nam, G.E.; Seo, J.A.; Yoon, T.; Seo, I.; Lee, J.H.; Im, D.; Bahn, K.-N.; Jeong, S.A.; Kang, T.S.; et al. Nut consumption has favorable effects on lipid profiles of Korean women with metabolic syndrome. Nutr. Res. 2014, 34, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D. Atherogenesis in perspective: Hypercholesterolemia and inflammation as partners in crime. Nat. Med. 2002, 8, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, Y.; Oka, Y.; Katagiri, H. Circulating oxidized LDL: A biomarker and a pathogenic factor. Curr. Opin. Lipidol. 2009, 20, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Cushman, M.; Stampfer, M.J.; Tracy, R.P.; Hennekens, C.H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N. Engl. J. Med. 1997, 336, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Sims, G.P.; Rowe, D.C.; Rietdijk, S.T.; Herbst, R.; Coyle, A.J. HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 2010, 28, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmiel-Haggai, M.; Cederbaum, A.I.; Nieto, N. A high-fat diet leads to the progression of non-alcoholic fatty liver disease in obese rats. FASEB J. 2004. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, M.J.; Cooper, J.N.; Erario, M.; Cheifetz, C.E. Pistachio nut consumption and serum lipid levels. J. Am. Coll. Nutr. 2007, 26, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.; Kwaw, I.; Matud, J.; Kurtz, I. Effect of pistachio nuts on serum lipid levels in patients with moderate hypercholesterolemia. J. Am. Coll. Nutr. 1999, 18, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Maranhão, P.A.; Kraemer-Aguiar, L.G.; de Oliveira, C.L.; Kuschnir, M.C.; Vieira, Y.R.; Souza, M.G.; Koury, J.C.; Bouskela, E. Brazil nuts intake improves lipid profile, oxidative stress and microvascular function in obese adolescents: A randomized controlled trial. Nutr. Metab. 2011, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Liu, Y.; Lv, X.; Yang, W. Effects of pistachios on body weight in Chinese subjects with metabolic syndrome. Nutr. J. 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Fitó, M. Effect of a traditional mediterranean diet on lipoprotein oxidation: a randomized controlled trial. Arch. Intern. Med. 2007, 167, 1195. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D.; Gebauer, S.K.; West, S.G.; Kris-Etherton, P.M. pistachios increase serum antioxidants and lower serum oxidized-ldl in hypercholesterolemic adults. J. Nutr. 2010, 140, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Jensen-Urstad, A.P.; Semenkovich, C.F. Fatty acid synthase and liver triglyceride metabolism: Housekeeper or messenger? Biochim. Biophys. Acta 2012, 1821, 747–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, Y.; DeBose-Boyd, R.A. Control of cholesterol synthesis through regulated ER-associated degradation of HMG CoA reductase. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 185–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Surra, J.C.; Barranquero, C.; Torcal, M.P.; Orman, I.; Segovia, J.C.; Guillén, N.; Navarro, M.A.; Arnal, C.; Osada, J. In comparison with palm oil, dietary nut supplementation delays the progression of atherosclerotic lesions in female apoE-deficient mice. Br. J. Nutr. 2013, 109, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Alturfan, A.A.; Emekli-Alturfan, E.; Uslu, E. Consumption of pistachio nuts beneficially affected blood lipids and total antioxidant activity in rats fed a high-cholesterol diet. Folia Biol. 2009, 55, 132–136. [Google Scholar] [PubMed]
- Kocyigit, A.; Koylu, A.A.; Keles, H. Effects of pistachio nuts consumption on plasma lipid profile and oxidative status in healthy volunteers. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Casas-Agustench, P.; Bulló, M.; Salas-Salvadó, J. Nuts, inflammation and insulin resistance. Asia Pac. J. Clin. Nutr. 2010, 19, 124–130. [Google Scholar] [PubMed]
- Koenig, W.; Sund, M.; Fröhlich, M.; Fischer, H.G.; Löwel, H.; Döring, A.; Hutchinson, W.L.; Pepys, M.B. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: Results from the MONICA (monitoring trends and determinants in cardiovascular disease) Augsburg Cohort Study, 1984 to 1992. Circulation 1999, 99, 237–242. [Google Scholar] [CrossRef]
- Yao, H.-C.; Zhao, A.-P.; Han, Q.-F.; Wu, L.; Yao, D.-K.; Wang, L.-X. Correlation between serum high-mobility group box-1 levels and high-sensitivity C-reactive protein and troponin I in patients with coronary artery disease. Exp. Ther. Med. 2013, 6, 121–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leclerc, P.; Wähämaa, H.; Idborg, H.; Jakobsson, P.J.; Harris, H.E.; Korotkova, M. IL-1β/HMGB1 complexes promote the pge2 biosynthesis pathway in synovial fibroblasts. Scand. J. Immunol. 2013, 77, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.; Perrone, A.; Attanzio, A.; Tesoriere, L.; Livrea, M.A. Sicilian pistachio (Pistacia vera L.) nut inhibits expression and release of inflammatory mediators and reverts the increase of paracellular permeability in IL-1β-exposed human intestinal epithelial cells. Eur. J. Nutr. 2015, 54, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Paterniti, I.; Impellizzeri, D.; Cordaro, M.; Siracusa, R.; Bisignano, C.; Gugliandolo, E.; Carughi, A.; Esposito, E.; Mandalari, G.; Cuzzocrea, S. The anti-inflammatory and antioxidant potential of pistachios (Pistacia vera L.) in vitro and in vivo. Nutrients 2017, 9, 915. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.; Allegra, M.; Angileri, F.; Pintaudi, A.M.; Livrea, M.A.; Tesoriere, L. Polymeric proanthocyanidins from Sicilian pistachio (Pistacia vera L.) nut extract inhibit lipopolysaccharide-induced inflammatory response in RAW 264.7 cells. Eur. J. Nutr. 2012, 51, 353–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ros, E.; Tapsell, L.C.; Sabaté, J. Nuts and berries for heart health. Curr. Atheroscler. Rep. 2010, 12, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Yu, H.; He, F.; Reilly, K.H.; Zhang, J.; Li, S.; Zhang, T.; Wang, B.; Ding, Y.; Xi, B. Nut consumption in relation to cardiovascular disease risk and type 2 diabetes: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2014, 100, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Del Gobbo, L.C.; Falk, M.C.; Feldman, R.; Lewis, K.; Mozaffarian, D. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: Systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am. J. Clin. Nutr. 2015, 102, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, N.; Aksoy, M.; Bagci, C.; Gergerlioglu, H.S.; Celik, H.; Herken, E.; Yaman, A.; Tarakcioglu, M.; Soydinc, S.; Sari, I.; et al. Pistachio intake increases high density lipoprotein levels and inhibits low-density lipoprotein oxidation in rats. Tohoku J. Exp. Med. 2007, 212, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.Y.; Beidler, J.; Hooshmand, S.; Figueroa, A.; Kern, M. Watermelon and L-arginine consumption improve serum lipid profile and reduce inflammation and oxidative stress by altering gene expression in rats fed an atherogenic diet. Nutr. Res. 2018, 58, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Bolling, B.W. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br. J. Nutr. 2015, 113, S68–S78. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.A.; Nyström, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and their derivatives: Structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef] [PubMed]
- Ros, E. Health Benefits of Nut Consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds used in the study are available from the authors. |




| Control | Pistachios | Mixed Nuts | |
|---|---|---|---|
| Initial body weight (g) | 49.29 ± 1.11 | 49.77 ± 0.88 | 49.85 ± 0.84 |
| Final body weight (g) | 369.4 ± 4.49 | 377.6 ± 4.95 | 378.3 ± 7.86 |
| Weight gain (g) | 320.1 ± 5.08 | 327.8 ± 4.37 | 328.4 ± 7.61 |
| Food intake (g/d) | 18.21 ± 0.46 | 18.79 ± 0.40 | 20.02 ± 0.65 |
| Water intake (g/d) | 26.18 ± 0.91 | 27.38 ± 1.28 | 26.79 ± 1.23 |
| Liver weight (g) | 22.11 ± 0.62 | 21.21 ± 0.86 | 22.00 ± 0.87 |
| Spleen weight (g) | 1.64 ± 0.13 | 1.59 ± 0.11 | 1.47 ± 0.17 |
| Epididymal fat weight (g) | 3.67 ± 0.14 | 3.56 ± 0.17 | 3.41 ± 0.15 |
| Control | Pistachios | Mixed Nuts | |
|---|---|---|---|
| AST (U/L) | 40.29 ± 3.94 a | 34.51 ± 0.53 a,b | 31.00 ± 1.88 b |
| ALT (U/L) | 28.16 ± 1.66 | 29.86 ± 2.29 | 33.19 ± 2.41 |
| ALP (U/L) | 63.35 ± 2.77 | 58.78 ± 2.54 | 64.32 ± 2.50 |
| LDH (U/L) | 97.44 ± 9.63 | 116.9 ± 17.8 | 108.3 ± 7.19 |
| CK (U/L) | 633.2 ± 64.9 | 600.6 ± 96.7 | 656.8 ± 57.1 |
| Ingredient (g) | Control | Pistachios | Mixed Nuts |
|---|---|---|---|
| Cornstarch | 14.30 | 13.40 | 13.50 |
| Sucrose | 33.00 | 32.40 | 32.70 |
| Cellulose | 5.00 | 4.10 | 4.50 |
| Casein | 20.00 | 18.30 | 18.70 |
| Corn oil | 5.00 | 5.00 | 5.00 |
| Milk fat | 16.00 | 12.40 | 11.70 |
| Cholesterol | 1.00 | 1.00 | 1.00 |
| Salt mix | 3.50 | 3.50 | 3.50 |
| Vitamin mix | 1.00 | 1.00 | 1.00 |
| DL-Methionine | 0.30 | 0.30 | 0.30 |
| Sodium cholate | 0.50 | 0.50 | 0.50 |
| Choline bitartrate | 0.40 | 0.40 | 0.40 |
| TBHQ | 0.003 | 0.003 | 0.003 |
| Pistachios | 0.00 | 8.10 | 0.00 |
| Mixed nuts | 0.00 | 0.00 | 7.50 |
| Total | 100.00 | 100.00 | 100.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, M.Y.; Groven, S.; Marx, A.; Rasmussen, C.; Beidler, J. Anti-Inflammatory, Antioxidant, and Hypolipidemic Effects of Mixed Nuts in Atherogenic Diet-Fed Rats. Molecules 2018, 23, 3126. https://doi.org/10.3390/molecules23123126
Hong MY, Groven S, Marx A, Rasmussen C, Beidler J. Anti-Inflammatory, Antioxidant, and Hypolipidemic Effects of Mixed Nuts in Atherogenic Diet-Fed Rats. Molecules. 2018; 23(12):3126. https://doi.org/10.3390/molecules23123126
Chicago/Turabian StyleHong, Mee Young, Shauna Groven, Amanda Marx, Caitlin Rasmussen, and Joshua Beidler. 2018. "Anti-Inflammatory, Antioxidant, and Hypolipidemic Effects of Mixed Nuts in Atherogenic Diet-Fed Rats" Molecules 23, no. 12: 3126. https://doi.org/10.3390/molecules23123126