Characterization and Prebiotic Potential of Longan Juice Obtained by Enzymatic Conversion of Constituent Sucrose into Fructo-Oligosaccharides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical Properties of Longan Juice
2.2. Chemical Composition of Longan Juice
2.3. Ethanol-Soluble Sugars Profile in Longan Juice
2.4. Molecular Size Distribution of Water-Soluble Polysaccharides
2.5. Monosaccharide and Uronic Acid Composition of Water-Soluble Polysaccharide and Insoluble Fiber
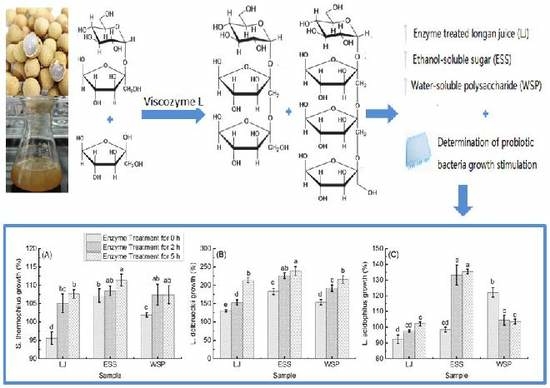
2.6. The Growth Effect of Treated Longan Juice on Probiotic Bacteria
3. Materials and Methods
3.1. Materials
3.2. Enzymatic Treatment of Longan Pulp Samples
3.3. Total Soluble Solid Content, pH, Yield, Clarity and Color Measurement
3.4. Proximate Composition of the Juice
3.5. Quantitative Analysis of Ethanol-Soluble Sugars using HPLC
3.6. Evaluation of the Molecular Size Changes of Water-Soluble Polysaccharides using High-Performance Gel Permeation Chromatography (GPC)
3.7. Monosaccharide Composition of Water-Soluble Polysaccharide and Insoluble Fiber
3.8. The Growth Effect of Longan Juice on Probiotic Bacteria
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Qiu, D.L. Longan Production and Research in China. Acta Hortic. 2014, 1029, 39–46. [Google Scholar] [CrossRef]
- Zheng, G.M.; Xu, L.X.; Wu, P.; Xie, H.H.; Jiang, Y.M.; Chen, F.; Wei, X.Y. Polyphenols from longan seeds and their radical-scavenging activity. Food Chem. 2009, 116, 433–436. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, G.; Wen, Y.; Liu, S.; Li, C.; Yang, R.; Li, W. Intestinal microbiota are involved in the immunomodulatory activities of longan polysaccharide. Mol. Nutr. Food Res. 2017, 61, 1700466. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Lin, C.; Chou, C.; Hsu, C. The effect of Longan seed polyphenols on colorectal carcinoma cells. Eur. J. Clin. Investig. 2010, 40, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Jiang, Y.; Wang, R. Ultra-high pressure treatment effects on polysaccharides and lignins of longan fruit pericarp. Food Chem. 2009, 112, 428–431. [Google Scholar] [CrossRef]
- Zhou, M.L.; Ndeurumio, K.H.; Zhao, L.; Hu, Z.Y. Impact of Precooling and Controlled-Atmosphere Storage on γ-Aminobutyric Acid (GABA) Accumulation in Longan (Dimocarpus longan Lour.) Fruit. J. Agric. Food Chem. 2016, 64, 6443–6450. [Google Scholar] [CrossRef] [PubMed]
- Kunworarath, N.; Rangkadilok, N.; Suriyo, T. Longan (Dimocarpus longan Lour.) inhibits lipopolysaccharide-stimulated nitric oxide production in macrophages by suppressing NF-κB and AP-1 signaling pathways. J. Ethnopharmacol. 2016, 179, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Dal Magro, L.; Goetze, D.; Ribeiro, C.T.; Paludo, N.; Rodrigues, E.; Hertz, P.F.; Klein, M.P.; Rodrigues, R.C. Identification of bioactive compounds from vitis labrusca L. variety concord grape juice treated with commercial enzymes: Improved yield and quality parameters. Food Bioprocess Technol. 2016, 9, 365–377. [Google Scholar] [CrossRef]
- Toushik, S.H.; Lee, K.; Lee, J. Functional Applications of Lignocellulolytic Enzymes in the Fruit and Vegetable Processing Industries. J. Food Sci. 2017, 82, 585–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Lee, H.; Park, Y.; Ra, K.S.; Shin, K.; Yu, K.; Suh, H.J. Mucilage removal from cactus cladodes (Opuntia humifusa Raf.) by enzymatic treatment to improve extraction efficiency and radical scavenging activity. LWT Food Sci. Technol. 2013, 51, 337–342. [Google Scholar] [CrossRef]
- Willems, J.L.; Low, N.H. Oligosaccharide formation during commercial pear juice processing. Food Chem. 2016, 204, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, W.; Liu, L. Physico-chemical properties of longan fruit during development and ripening. Sci. Hortic. 2016, 207, 160–167. [Google Scholar] [CrossRef]
- Te, M.L.; Mallard, S.; Mann, J. Dietary sugars and body weight: Systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2012, 346, 1–25. [Google Scholar] [CrossRef]
- Johansson, S.; Diehl, B.; Christakopoulos, P.; Austin, S.; Vafiadi, C. Oligosaccharide synthesis in fruit juice concentrates using a glucansucrase from Lactobacillus reuteri 180. Food Bioprod. Process. 2016, 98, 201–209. [Google Scholar] [CrossRef]
- Vega-Paulino, R.J.; Zúniga-Hansen, M.E. Potential application of commercial enzyme preparations for industrial production of short-chain fructooligosaccharides. J. Mol. Catal. B Enzym. 2012, 76, 44–51. [Google Scholar] [CrossRef]
- Zeng, X.; Zhou, K.; Liu, D.; Brennan, C.S.; Brennan, M.; Zhou, J.; Yu, S. Preparation of fructooligosaccharides using Aspergillus niger 6640 whole-cell as catalyst for bio-transformation. LWT-Food Sci. Technol. 2016, 65, 1072–1079. [Google Scholar] [CrossRef]
- Maiorano, A.E.; Piccoli, R.M.; Da Silva, E.S.; de Andrade Rodrigues, M.F. Microbial production of fructosyltransferases for synthesis of pre-biotics. Biotechnol. Lett. 2008, 30, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Nam, S.; Kang, H. Synthesis of thermo-and acid-stable novel oligosaccharides by using dextransucrase with high concentration of sucrose. Enzym. Microb. Technol. 2007, 40, 1117–1123. [Google Scholar] [CrossRef]
- Endo, A.; Nakamura, S.; Konishi, K. Variations in prebiotic oligosaccharide fermentation by intestinal lactic acid bacteria. Int. J. Food Sci. Nutr. 2016, 62, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Delgadoa, G.T.C.; Tamashiro, W.M. Role of prebiotics in regulation of microbiota and prevention of obesity. Food Res. Int. 2018, 113, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, A.L.; Rodrigues, L.R.; Lima, N.M.; Teixeira, J.A. An overview of the recent developments on fructooligosaccharide production and applications. Food Bioprocess Technol. 2014, 7, 324–337. [Google Scholar] [CrossRef] [Green Version]
- Bryk, G.; Coronel, M.Z.; Lugones, C.; Mandalunis, P.; Rio, M.E.; Gualtieri, A.F.; de Portela, M.L.P.M.; Zeni, S.N. Effect of a mixture of GOS/FOS® on calcium absorption and retention during recovery from protein malnutrition: Experimental model in growing rats. Eur. J. Nutr. 2016, 55, 2445–2458. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Lu, J.; Li, B. Effect of functional oligosaccharides and ordinary dietary fiber on intestinal microbiota diversity. Front. Microbiol. 2017, 8, 1750. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.H.; Seo, Y.; Cho, J.; Lee, S.; Kim, G.J.; Yoon, J.W.; Ahn, S.H.; Hwang, K.H.; Park, J.S.; Jang, T.S.; et al. Synthesis of oligosaccharide-containing orange juice using glucansucrase. Biotechnol. Bioprocess Eng. 2015, 20, 447–452. [Google Scholar] [CrossRef]
- Stéger-Máté, M.; Horváth-Kerkai, E. Manufacturing fruit beverages and concentrates. In Handbook of Fruits and Fruit Processing, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 215–228. [Google Scholar]
- Tapre, A.R. Pectinases: Enzymes for fruit processing industry. Int. Food Res. J. 2014, 21, 447–453. [Google Scholar]
- Mrabet, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Hamza, H.; Rodríguez-Arcos, R.; Guillén-Bejarano, R.; Sindic, M.; Jiménez-Araujo, A. Enzymatic conversion of date fruit fiber concentrates into a new product enriched in antioxidant soluble fiber. LWT-Food Sci. Technol. 2017, 75, 727–734. [Google Scholar] [CrossRef] [Green Version]
- Dongowski, G.; Sembries, S. Effects of commercial pectolytic and cellulolytic enzyme preparations on the apple cell wall. J. Agric. Food Chem. 2001, 49, 4236–4242. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, N.; Trollope, K.M.; Steenkamp, E.T.; Wingfield, B.D.; Volschenk, H. Identification of the gene for beta-fructofuranosidase from Ceratocystis moniliformis CMW 10134 and characterization of the enzyme expressed in Saccharomyces cerevisiae. BMC Biotechnology 2013, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Rostami, H.; Gharibzahedi, S.M.T. Cellulase-assisted extraction of polysaccharides from Malva sylvestris: Process optimization and potential functionalities. Int. J. Biol. Macromol. 2017, 101, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gutierrez, F.; Ratering, S.; Juárez-Flores, B.; Godinez-Hernandez, C.; Geissler-Plaum, R.; Prell, F.; Zorn, H.; Czermak, P.; Schnell, S. Potential use of Agave salmiana as a prebiotic that stimulates the growth of probiotic bacteria. LWT-Food Sci. Technol. 2017, 84, 151–159. [Google Scholar] [CrossRef]
- Velasquez, M.T. Altered Gut Microbiota: A Link Between Diet and the Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2018, 16, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Aliaa, A.R.N.; Mazlina, M.K.S.; Taip, F.S. Response surface optimization for clarification of white pitaya juice using a commercial enzyme. J. Food Process Eng. 2010, 2, 333–347. [Google Scholar] [CrossRef]
- Sin, H.N.; Yusof, S.; Hamid, N.; Rahman, R.A. Optimization of enzymatic clarification of sapodilla juice using response surface methodology. J. Food Eng. 2006, 73, 313–319. [Google Scholar] [CrossRef]
- Jeong, I.Y.; Lee, H.J.; Park, Y.D.; Jin, C.H.; Choi, D.S.; Byun, M.W. Effects of gamma irradiation on total polyphenols, radical scavenging activities and decolourization of Nelumbo nucifera extracts. Radiat. Phys. Chem. 2009, 78, 575–577. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Menkovska, M.; Levkov, V.; Damjanovski, D.; Gjorgovska, N.; Knezevic, D.; Nikolova, N.; Andreevska, D. Content of TDF, SDF and IDF in cereals grown by organic and conventional farming—A short report. Pol. J. Food Nutr. Sci. 2017, 67, 241–244. [Google Scholar] [CrossRef]
- Kurita, O.; Miyake, Y.; Yamazaki, E. Chemical modification of citrus pectin to improve its dissolution into water. Carbohydr Polym. 2012, 87, 1720–1727. [Google Scholar] [CrossRef]
- Chaikham, P.; Rattanasena, P.; Phunchaisri, C. Quality changes of litchi (Litchi chinensis Sonn.) in syrup due to thermal and high pressure processes. LWT Food Sci. Technol. 2017, 75, 751–760. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nowak, R.; Nowacka-Jechalke, N.; Juda, M.; Malm, A. The preliminary study of prebiotic potential of Polish wild mushroom polysaccharides: The stimulation effect on Lactobacillus strains growth. Eur. J. Nutr. 2017, 54, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |





| Treated Time (h) | Yield (%) | Clarity (T/%) | Total Solid Content (°Brix) | Color Parameters | ||
|---|---|---|---|---|---|---|
| L * | A * | B * | ||||
| 0 | 76.91 ± 3.23 c | 45.80 ± 2.07 c | 19.33 ± 0.06 c | 52.81 ± 0.17 a | 8.28 ± 0.03 c | 12.90 ± 0.19 b |
| 2 | 85.78 ± 1.82 b | 66.27 ± 2.92 b | 19.87 ± 0.06 b | 49.41 ± 0.39 b | 8.63 ± 0.04 b | 14.21 ± 0.10 a |
| 5 | 93.03 ± 1.95 a | 82.07 ± 1.15 a | 20.13 ± 0.06 a | 47.65 ± 0.09 c | 9.01 ± 0.16 a | 14.04 ± 0.12 a |
| Treated Time (h) | Ethanol-Soluble Sugars (mg/g) | Water-Soluble Polysaccharides (mg/g) | Insoluble Fiber (mg/g) | Water-Soluble Pectin (mg/g) | Polyphenols (µg/g) | Soluble Protein (mg/g) |
|---|---|---|---|---|---|---|
| 0 | 175.78 ± 0.94 a | 3.61 ± 0.15 c | 11.74 ± 0.49 a | 0.07 ± 0.00 c | 12.23 ± 0.15 a | 3.06 ± 0.05 a |
| 2 | 175.82 ± 1.48 a | 4.88 ± 0.16 b | 8.98 ± 0.15 b | 0.16 ± 0.00 b | 10.99 ± 0.11 b | 1.02 ± 0.01 b |
| 5 | 175.92 ± 1.68 a | 8.30 ± 0.27 a | 6.07 ± 0.04 c | 0.20 ± 0.01 a | 8.69 ± 0.29 c | 0.93 ± 0.01 c |
| Treated Time (h) | Fructose (mg/g) | Glucose (mg/g) | Sucrose (mg/g) | 1-Kestose (mg/g) | Nystose (mg/g) | FOSs (mg/g) |
|---|---|---|---|---|---|---|
| 0 | 27.92 ± 0.08 a | 36.87 ± 0.06 c | 110.98 ± 0.80 a | 0 | 0 | 0 |
| 2 | 27.27 ± 0.20 b | 56.89 ± 0.51 b | 42.25 ± 0.38 b | 42.25 ± 0.68 b | 7.16 ± 0.18 b | 49.40 ± 0.58 b |
| 5 | 28.17 ± 0.23 a | 69.05 ± 0.30 a | 17.11 ± 0.26 c | 47.52 ± 0.51 a | 14.08 ± 0.45 a | 61.59 ± 0.93 a |
| Treated Time (h) | Fraction 1 (%) | Fraction 2 (%) | Fraction 3 (%) | Fraction 4 (%) | Fraction 5 (%) |
|---|---|---|---|---|---|
| 0 | 17.67 ± 0.61 a | 4.87 ± 0.41 a | 2.22 ± 0.04 c | 55.12 ± 2.12 a | 20.13 ± 1.05 c |
| 2 | 13.91 ± 0.46 b | 4.64 ± 0.27 a | 20.32 ± 0.28 b | 30.05 ± 0.05 b | 31.10 ± 0.14 b |
| 5 | 9.56 ± 0.13 c | 4.11 ± 0.07 b | 31.47 ± 0.20 a | 18.33 ± 0.36 c | 36.43 ± 0.04 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Lan, H.; Zhao, L.; Wang, K.; Hu, Z. Characterization and Prebiotic Potential of Longan Juice Obtained by Enzymatic Conversion of Constituent Sucrose into Fructo-Oligosaccharides. Molecules 2018, 23, 2596. https://doi.org/10.3390/molecules23102596
Cheng Y, Lan H, Zhao L, Wang K, Hu Z. Characterization and Prebiotic Potential of Longan Juice Obtained by Enzymatic Conversion of Constituent Sucrose into Fructo-Oligosaccharides. Molecules. 2018; 23(10):2596. https://doi.org/10.3390/molecules23102596
Chicago/Turabian StyleCheng, Yongxia, Haibo Lan, Lei Zhao, Kai Wang, and Zhuoyan Hu. 2018. "Characterization and Prebiotic Potential of Longan Juice Obtained by Enzymatic Conversion of Constituent Sucrose into Fructo-Oligosaccharides" Molecules 23, no. 10: 2596. https://doi.org/10.3390/molecules23102596