A Century of Vaccine Adjuvants: From 1920 to 2020 and Beyond
A special issue of Vaccines (ISSN 2076-393X). This special issue belongs to the section "Vaccine Adjuvants".
Deadline for manuscript submissions: closed (31 May 2022) | Viewed by 8760
Special Issue Editor
Interests: antitumor vaccines; adjuvants; adaptive immunity; eosinophils; checkpoint blockade; allergy
Special Issues, Collections and Topics in MDPI journals
Special Issue Information
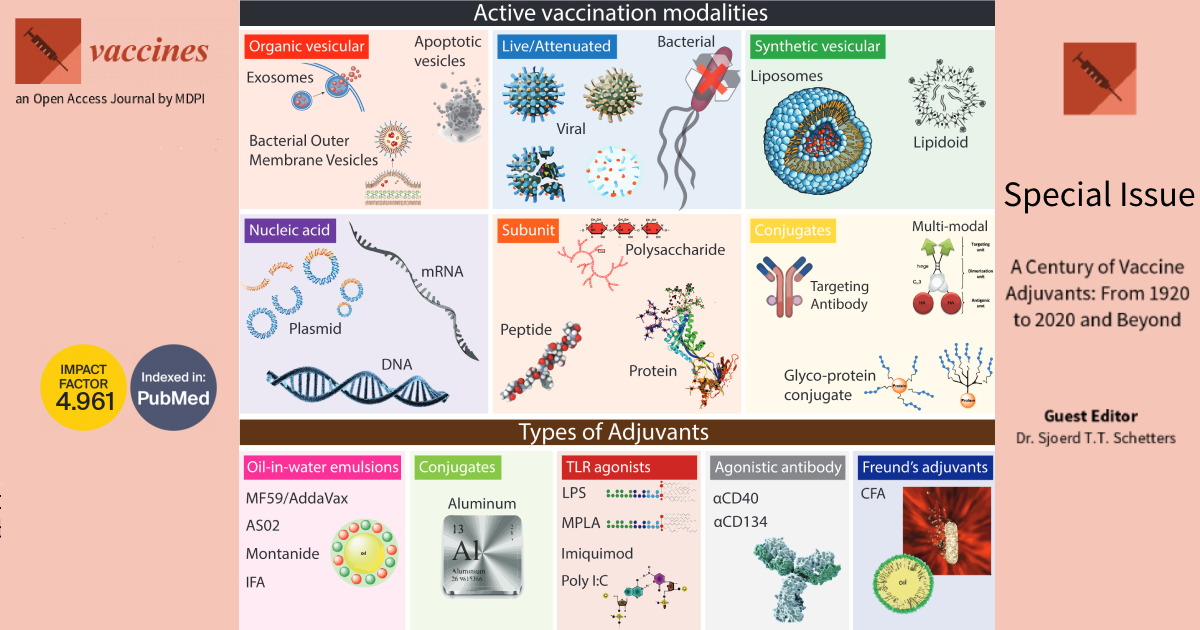
Dear colleagues,
In the 20th century, science has come a long way in understanding protective immunity and its practical use in vaccinology. From the efficacy of Jenner’s variolation, through the discovery of inactivated biological sources as vaccine compounds by Louis Pasteur and Calmette and Guerin, to the development of tuberculosis, diphtheria, tetanus, and whooping cough vaccines in 1920-30, practical immunology has proven vital to the ultimate prosperity of humanity. As our understanding of adaptive immunity increases, there is a growing urge to define specific modes of action that result in long-term protective immunity. In principle, vaccines contain antigen and innate immune activators to satisfy the preconditions of adaptive immunity. The delivery of antigens can be highly defined (for example, synthetic peptides or mRNA) or included by association (for example, live/attenuated pathogens). However, not all antigen modalities used in vaccination elicit the innate immune activation needed for downstream adaptive immunity. Instead, these antigens are mixed with or conjugated to immunogenic compounds, called adjuvants, capable of activating dendritic cells. A wide variety of adjuvants have now been described, designed, and used in clinical settings (see figure). With the advent of new vaccines, further pushed to the clinic because of the current COVID-19 pandemic, there is a need to understand the immune stimulatory capacity of adjuvants in vaccine formulations. In this issue of Vaccines, we will explore these concepts further and provide a collection of excellent scientific manuscripts aimed at increasing our understanding of vaccinology.
Dr. Sjoerd T.T. Schetters
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Vaccines is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- vaccine adjuvants
- vaccine formulations
- vaccine development






