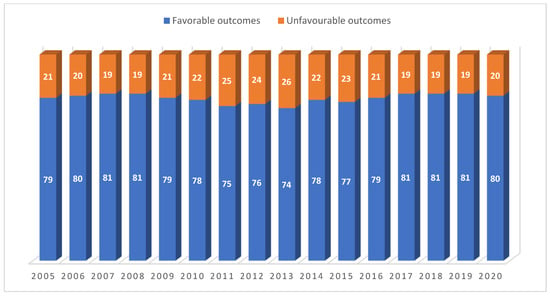
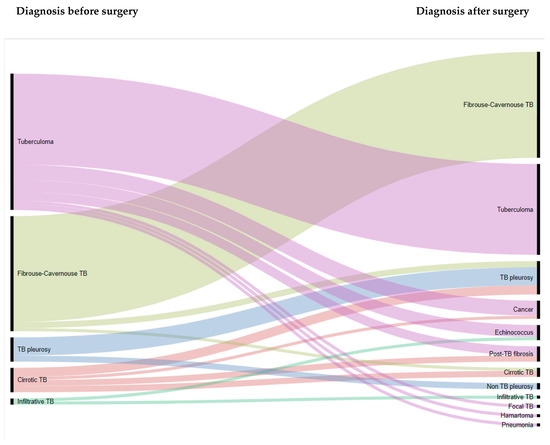
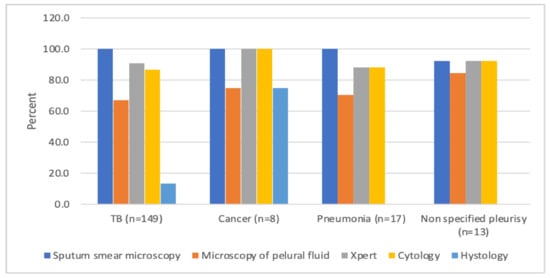
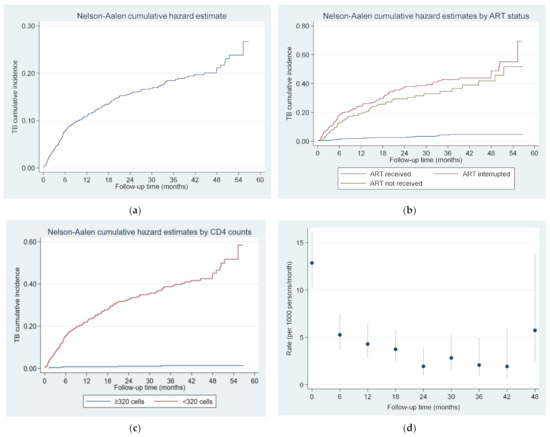
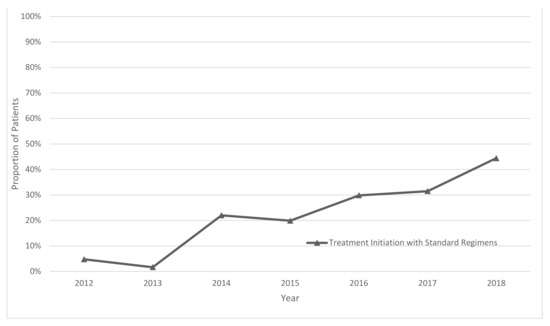
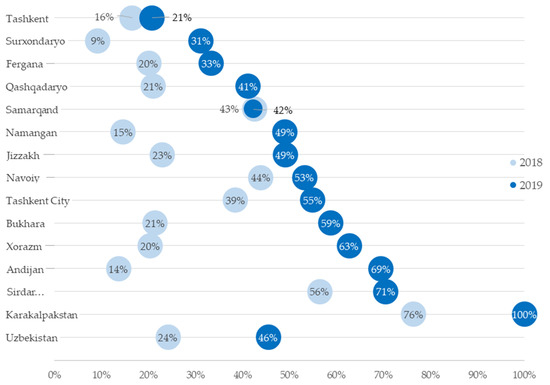
Operational Research to Inform Programmatic Approaches to Management of Tuberculosis in Uzbekistan (Closed)
A project collection of International Journal of Environmental Research and Public Health (ISSN 1660-4601).
Papers displayed on this page all arise from the same project. Editorial decisions were made independently of project staff and handled by the Editor-in-Chief or qualified Editorial Board members.
Viewed by 32590Editors
Interests: emerging infectious diseases; infection prevention and control; climate change and vector borne diseases; HIV; viral Hepatitis; sexually transmitted diseases; tuberculosis
Interests: tuberculosis; pulmonary diseases, research area; heath care managemnet
Interests: drug-resistant tuberculosis; tuberculosis prevention and treatment; tuberculosis and viral Hepatitis co-infection; tuberculosis and HIV co-infection; operational research
Interests: public health; health system strenghtening; governance; coordination and partnerships; universal health coverage; emergency preparedness and response and healthy life styles and behavior
Project Overview
Dear Colleagues,
The European Tuberculosis Research Initiative (ERI-TB) was launched by the WHO Regional Office for Europe in 2017 in order to advance TB research in the region, and is one of the platforms that supports the implementation of the TB Action Plan of 2016–2020. Responsible officers within the Joint Tuberculosis, HIV, and Viral Hepatitis Program serve as ERI-TB secretariats. One of the ERI-TB objectives was to define a regional TB research agenda. The ERI-TB secretariats have facilitated the process and a European TB Research Agenda (ERA-TB) was developed using the core Regional (i) technical expertise, (ii) engagement of the civil society, as well as (iii) consultation with national and (iv) regional counterparts.
In June 2018, the WHO Regional Office for Europe launched the first Structured Operational Research Training of ERI-TB (SORT-TB) to facilitate the implementation of ERA-TB. The aim of SORT-TB is to build the capacity of TB researchers in East Europe and Central Asia in partnership with UNICEF/UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases (TDR). The first cohort of SORT-TB took the concept of the TDR’s SORT IT course (https://www.who.int/tdr/capacity/strengthening/sort/en/) to the next level by (i) supporting the implementation of ERA-TB, (ii) acting within the framework of the bilateral collaborative agreements (BCA) with member states, and (iii) supporting national counterparts to adapt country TB policies by turning research into action. The SORT-TB course consists of three workshops, taking the principal investigators (PIs) through the process of preparing an OR protocol, analyzing data, and preparing publishable manuscripts. In 2019–2020, the WHO Country Office in Uzbekistan, together with the WHO Regional Office for Europe, implemented the first country-specific SORT-TB course.
Thirteen research projects were carried out within the first country-specific SORT-TB cohort. Principal investigators from Uzbekistan, selected on a competitive basis, were enrolled into the course. Priority was given to those research projects that answer research questions defined in ERA-TB and that serve the purpose of improving the programmatic management of TB in Uzbekistan. Principal investigators were assigned into working groups, each consisting of two principal investigators, one senior mentor, one junior mentor, and one/two WHO co-mentors. Senior and junior mentors were selected from the pool of regional experts with experience in research and TB. Manuscripts prepared by research groups will serve the purpose of tailoring programs addressing the diagnostics, treatment, and care of TB in Uzbekistan.
The project collaborators are contributing work exclusively this Special Issue.
Dr. Masoud Dara
Dr. Nargiza Parpieva
Dr. Askar Yedilbayev
Dr. Lianne Kuppens
Dr. Jamshid Gadoev
Project Leaders
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. International Journal of Environmental Research and Public Health is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2500 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- tuberculosis
- multidrug- and rifampicin-resistance (MDR/RR-TB)
- extensively drug-resistant TB (XDR TB)
- Central Asia
- treatment success
- adverse events
- mycosis
- Xpert MTB/Rif Ultra
- Line probe
- assay (HAIN)
- sputum culture
- TB pleurisy
- urine from genital TB
- TB/HIV
- childhood TB