Emerging Therapeutics in Advanced Melanoma
A topical collection in Cancers (ISSN 2072-6694). This collection belongs to the section "Cancer Therapy".
Viewed by 19504Editors
Interests: melanoma; mohs surgery; immunotherapy; health disparities; clinical practice guidelines
Topical Collection Information
Dear Colleagues,
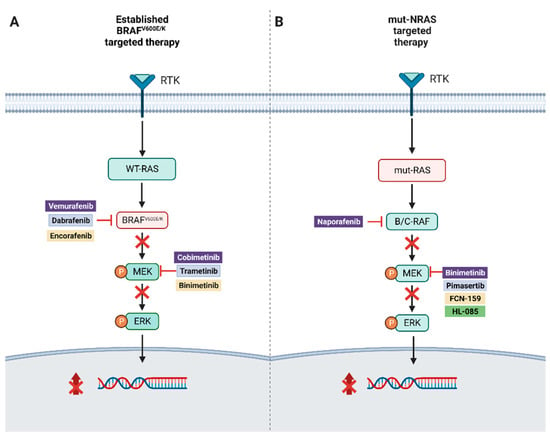
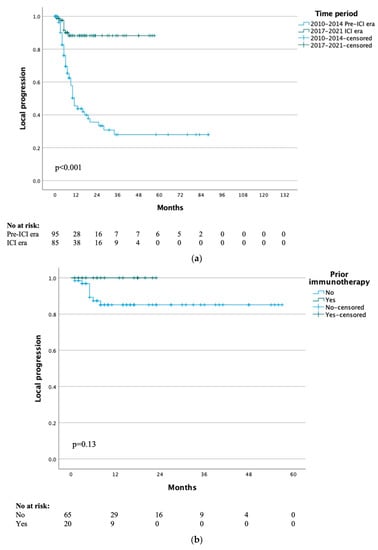
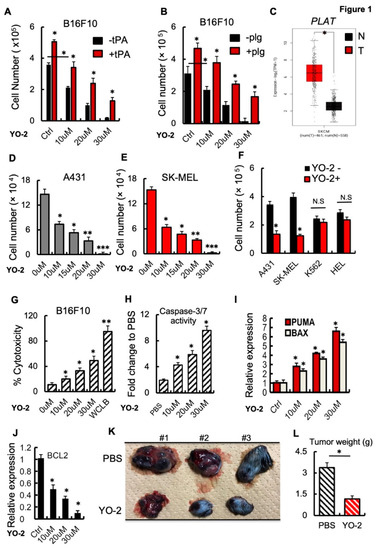
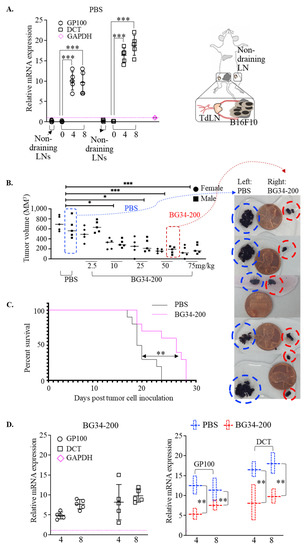
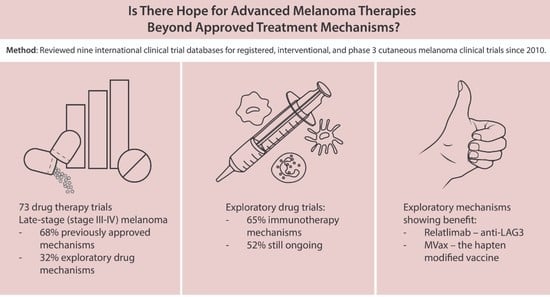
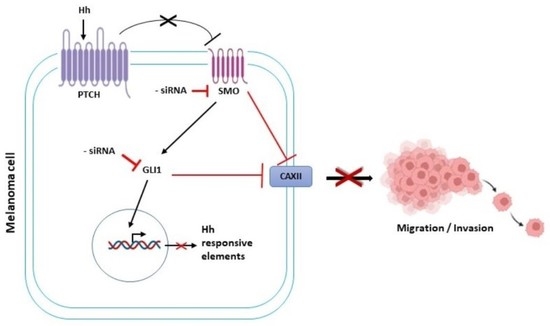
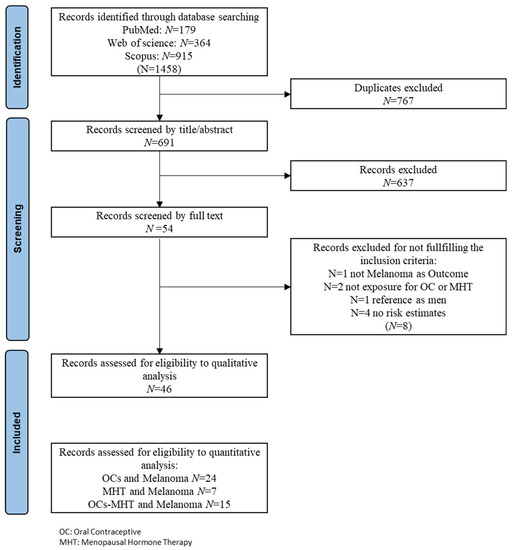
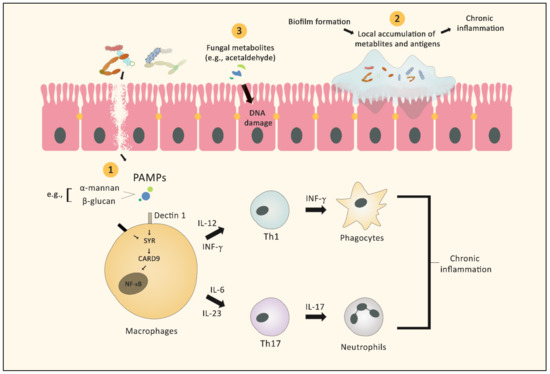
Treatment options for advanced melanoma have evolved rapidly over the past decade. Survival for patients with stage 4 disease has improved remarkably with the introduction of immune and targeted therapies. Our understanding of the immune system and its interaction with melanoma continues to evolve; gene therapies are becoming more refined; tumor and host metabolisms provide intriguing targets to overcome therapeutic resistance. Many efforts have led to current clinical trials, but nascent research that will define the next wave of melanoma therapies may yet be underappreciated.
This collection focuses on Emerging Therapeutics in Advanced Melanoma and seeks expert reviews as well as original research articles to highlight the most promising research in melanoma. We are seeking publications from research specialists in the melanoma microenvironment including immune subsets (i.e. T cells, macrophages, dendritic cells, etc.), stromal components (i.e. fibroblasts, endothelial cells, pericytes, etc.), and the nutrient microenvironment with its metabolic implications. Additional contributions will include emerging targets for drug therapies, improved uses of old treatments (i.e. chemotherapy, radiation, etc.), the evolution of oncolytic virotherapies, and novel approaches like gene or stem cell therapies. This work will ask experts to identify what they see as the most promising lines of investigation, and will provide a glimpse into the future of melanoma treatment.
We look forward to receiving your contributions.
Dr. Jeremy S. Bordeaux
Dr. Luke D. Rothermel
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- melanoma
- tumor microenvironment
- cancer metabolism
- immunotherapy
- targeted therapy
- oncolytic virotherapy
- chemotherapy resistance
- gene
- therapy
- stem cell therapy
- cancer vaccine