Trends in Melanoma Phase 3 Clinical Trials since 2010: Is there Hope for Advanced Melanoma Therapies beyond Approved Treatment Mechanisms?
Abstract
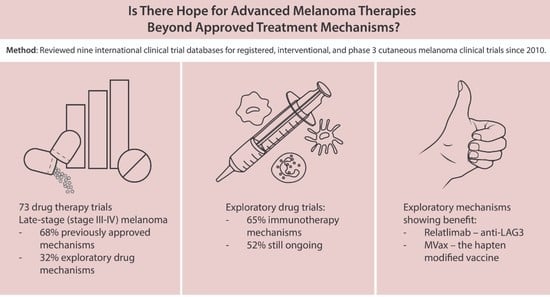
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Late-Stage Trials (Stage III and IV)
3.1.1. Approved vs. Exploratory Therapies
Locoregional: Approved vs. Exploratory
Systemic: Approved vs. Exploratory
Funding: Approved vs. Exploratory Trials
3.1.2. Trials Investigating Exploratory Drug Mechanisms with Positive Results
3.1.3. Treatment Context (Unresectable/Metastatic; Adjuvant; Neoadjuvant)
3.1.4. Combination Therapies
4. Discussion
4.1. Approved Mechanisms
4.2. Exploratory Mechanisms
4.3. Promising Therapeutics
4.4. Exploratory Drug Mechanisms Showing Positive Results
4.5. Exploratory Drug Mechanisms Showing Negative Results
4.6. Ongoing Trials
4.7. Opportunities for Progress
4.8. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Larkin, J.M.; Haanen, J.B.; Ribas, A.; Hogg, D.; Hamid, O.; Ascierto, P.A.; Testori, A.; et al. Updated overall survival (OS) results for BRIM-3, a phase III randomized, open-label, multicenter trial comparing BRAF inhibitor vemurafenib (vem) with dacarbazine (DTIC) in previously untreated patients with BRAF(V600E)-mutated melanoma. J. Clin. Oncol. 2012, 30, 8502. [Google Scholar] [CrossRef]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H., Jr.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2014, 372, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Andtbacka, R.H.I.; Collichio, F.; Harrington, K.J.; Middleton, M.R.; Downey, G.; Öhrling, K.; Kaufman, H.L. Final analyses of OPTiM: A randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III-IV melanoma. J. Immunother. Cancer 2019, 7, 145. [Google Scholar] [CrossRef] [Green Version]
- Robert, C.; Ribas, A.; Wolchok, J.D.; Hodi, F.S.; Hamid, O.; Kefford, R.; Weber, J.S.; Joshua, A.M.; Hwu, W.J.; Gangadhar, T.C.; et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet 2014, 384, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Long, G.V.; Fung, C.; Menzies, A.M.; Pupo, G.M.; Carlino, M.S.; Hyman, J.; Shahheydari, H.; Tembe, V.; Thompson, J.F.; Saw, R.P.; et al. Increased MAPK reactivation in early resistance to dabrafenib/trametinib combination therapy of BRAF-mutant metastatic melanoma. Nat. Commun. 2014, 5, 5694. [Google Scholar] [CrossRef]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggermont, A.M.; Chiarion-Sileni, V.; Grob, J.J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2015, 16, 522–530. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Chiarion-Sileni, V.; Grob, J.J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N. Engl. J. Med. 2016, 375, 1845–1855. [Google Scholar] [CrossRef] [Green Version]
- Weber, J.S.; Ascierto, P.A.; Middleton, M.R.; Hennicken, D.; Zoffoli, R.; Pieters, A.; Amadi, A.; Kupas, K.; Kotapati, S.; Moshyk, A.; et al. Indirect treatment comparison of nivolumab versus placebo as adjuvant treatment for resected melanoma. Eur. J. Cancer 2021, 158, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, O.J.; Kicinski, M.; Valpione, S.; Gandini, S.; Suciu, S.; Blank, C.U.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; et al. Prognostic and predictive value of β-blockers in the EORTC 1325/KEYNOTE-054 phase III trial of pembrolizumab versus placebo in resected high-risk stage III melanoma. Eur. J. Cancer 2022, 165, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Kirkwood, J.M.; Chiarion Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; et al. Five-Year Analysis of Adjuvant Dabrafenib plus Trametinib in Stage III Melanoma. N. Engl. J. Med. 2020, 383, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [Green Version]
- Gide, T.N.; Wilmott, J.S.; Scolyer, R.A.; Long, G.V. Primary and Acquired Resistance to Immune Checkpoint Inhibitors in Metastatic Melanoma. Clin. Cancer Res. 2018, 24, 1260–1270. [Google Scholar] [CrossRef] [Green Version]
- Eroglu, Z.; Ribas, A. Combination therapy with BRAF and MEK inhibitors for melanoma: Latest evidence and place in therapy. Ther. Adv. Med. Oncol. 2016, 8, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Umscheid, C.A.; Margolis, D.J.; Grossman, C.E. Key concepts of clinical trials: A narrative review. Postgrad. Med. 2011, 123, 194–204. [Google Scholar] [CrossRef]
- De Smedt, J.; Van Kelst, S.; Boecxstaens, V.; Stas, M.; Bogaerts, K.; Vanderschueren, D.; Aura, C.; Vandenberghe, K.; Lambrechts, D.; Wolter, P.; et al. Vitamin D supplementation in cutaneous malignant melanoma outcome (ViDMe): A randomized controlled trial. BMC Cancer 2017, 17, 562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwala, S.; Wachter, E. PV-10 Intralesional Injection vs Systemic Chemotherapy or Oncolytic Viral Therapy for Treatment of Locally Advanced Cutaneous Melanoma. Identifier NCT02288897. Available online: https://clinicaltrials.gov/ct2/show/NCT02288897 (accessed on 1 August 2022).
- National Library of Medicine (U.S.). Melablock: A Multicentre Randomized, Double—Blinded and Placebo—Controlled Clinical Trial on the Efficacy and Safety of Once Daily Propranolol 80 mg Retard for the Prevention of Cutaneous Malignant Melanoma Recurrence. Identifier NCT02962947. Available online: https://clinicaltrials.gov/ct2/show/NCT02962947 (accessed on 1 August 2022).
- Brożyna, A.A.; Hoffman, R.M.; Slominski, A.T. Relevance of Vitamin D in Melanoma Development, Progression and Therapy. Anticancer Res. 2020, 40, 473–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemeshow, S.; Sørensen, H.T.; Phillips, G.; Yang, E.V.; Antonsen, S.; Riis, A.H.; Lesinski, G.B.; Jackson, R.; Glaser, R. β-Blockers and survival among Danish patients with malignant melanoma: A population-based cohort study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2273–2279. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.F.; Agarwala, S.S.; Smithers, B.M.; Ross, M.I.; Scoggins, C.R.; Coventry, B.J.; Neuhaus, S.J.; Minor, D.R.; Singer, J.M.; Wachter, E.A. Phase 2 Study of Intralesional PV-10 in Refractory Metastatic Melanoma. Ann. Surg. Oncol. 2015, 22, 2135–2142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutzmer, R.; Stroyakovskiy, D.; Gogas, H.; Robert, C.; Lewis, K.; Protsenko, S.; Pereira, R.P.; Eigentler, T.; Rutkowski, P.; Demidov, L.; et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): Primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020, 395, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, L.; Livingstone, E.; Krackhardt, A.; Schultz, E.S.; Göppner, D.; Assaf, C.; Trebing, D.; Stelter, K.; Windemuth-Kieselbach, C.; Ugurel, S.; et al. Encorafenib, binimetinib plus pembrolizumab triplet therapy in patients with advanced BRAFV600 mutant melanoma: Safety and tolerability results from the phase I IMMU-TARGET trial. Eur. J. Cancer. 2021, 158, 72–84. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). A Phase 3, Randomized, Double-blind Study of Adjuvant Immunotherapy With Nivolumab + Relatlimab Fixed-dose Combination Versus Nivolumab Monotherapy After Complete Resection of Stage III-IV Melanoma. Identifier NCT05002569. Available online: https://clinicaltrials.gov/ct2/show/NCT05002569 (accessed on 1 August 2022).
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. RELATIVITY-047 Investigators. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Berd, D.; Sato, T.; Cohn, H.; Maguire HCJr Mastrangelo, M.J. Treatment of metastatic melanoma with autologous, hapten-modified melanoma vaccine: Regression of pulmonary metastases. Int. J. Cancer 2001, 94, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Berd, D.; Sato, T.; Maguire HCJr Kairys, J.; Mastrangelo, M.J. Immunopharmacologic analysis of an autologous, hapten-modified human melanoma vaccine. J. Clin. Oncol. 2004, 22, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Giacomantonio, C.A. The Response to Intralesional IL-2 and/or BCG Treatment for Cutaneous Metastatic Melanoma. Identifier NCT03928275. Available online: https://clinicaltrials.gov/ct2/show/NCT03928275 (accessed on 1 August 2022).
- Slingluff, C.L.; Lewis, K.D.; Andtbacka, R.; Hyngstrom, J.; Milhem, M.; Markovic, S.N.; Bowles, T.; Hamid, O.; Hernandez-Aya, L.; Claveau, J.; et al. Multicenter, double-blind, placebo-controlled trial of seviprotimut-L polyvalent melanoma vaccine in patients with post-resection melanoma at high risk of recurrence. J. Immunother. Cancer 2021, 9, e003272. [Google Scholar] [CrossRef]
- Javed, A.; Sato, S.; Sato, T. Autologous melanoma cell vaccine using monocyte-derived dendritic cells (NBS20/eltrapuldencel-T). Future Oncol. 2016, 12, 751–762. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J. A Randomized, Double--Blind, Placebo-Controlled Phase III Study to Evaluate Active Immunization in Adjuvant Therapy of Patients with Stage IIIB and IIIC Melanoma with Natural Dendritic Cells Pulsed With Synthetic Peptides. Identifier NCT02993315. Available online: https://www.clinicaltrials.gov/ct2/show/NCT02993315 (accessed on 1 August 2022).
- National Library of Medicine (U.S.). Allogeneic Vaccine Modified to Express HLA A2/4-1BB Ligand for High Risk or Low Residual Disease Melanoma Patients. Identifier NCT01861938. Available online: https://clinicaltrials.gov/ct2/show/NCT01861938 (accessed on 1 August 2022).
- Kjeldsen, J.W.; Lorentzen, C.L.; Martinenaite, E.; Ellebaek, E.; Donia, M.; Holmstroem, R.B.; Klausen, T.W.; Madsen, C.O.; Ahmed, S.M.; Weis-Banke, S.E.; et al. A phase 1/2 trial of an immune-modulatory vaccine against IDO/PD-L1 in combination with nivolumab in metastatic melanoma. Nat. Med. 2021, 27, 2212–2223. [Google Scholar] [CrossRef] [PubMed]
- John, B.A.G. Randomized Phase III Study Comparing a Non-myeloablative Lymphocyte Depleting Regimen of Chemotherapy Followed by Infusion of Tumor Infiltrating Lymphocytes and Interleukin-2 to Standard Ipilimumab Treatment in Metastatic Melanoma. Identifier NCT02278887. Available online: https://clinicaltrials.gov/ct2/show/NCT02278887 (accessed on 1 August 2022).
- Hodi, F.S. Randomized Phase II/III Study of Nivolumab Plus Ipilimumab Plus Sargramostim Versus Nivolumab Plus Ipilimumab in Patients With Unresectable Stage III or Stage IV Melanoma. Identifier NCT02339571. Available online: https://clinicaltrials.gov/ct2/show/NCT02339571 (accessed on 1 August 2022).
- GROB, J.J. A Prospective, Multicenter, Randomized, Open-Label, Active Controlled, Two-Parallel Groups, Phase 3 Study to Compare the Efficacy and Safety of Masitinib at 7.5 mg/kg/Day to Dacarbazine in the Treatment of Patients with Non-Resectable or Metastatic Stage 3 or Stage 4 Melanoma Carrying a Mutation in the Juxta Membrane Domain of C-Kit. Identifier NCT01280565. Available online: https://clinicaltrials.gov/ct2/show/NCT01280565 (accessed on 1 August 2022).
- National Library of Medicine (U.S.). A Phase 3 Randomized, Placebo-Controlled Trial to Evaluate the Safety and Efficacy of Pembrolizumab (MK-3475) and Lenvatinib (E7080/MK-7902) versus Pembrolizumab Alone as First-Line Intervention in Participants with Advanced Melanoma (LEAP-003). Identifier NCT03820986. Available online: https://clinicaltrials.gov/ct2/show/NCT03820986 (accessed on 1 August 2022).
- National Library of Medicine (U.S.). A Phase 3, Randomized, Double-Blind Study of BMS-986205 Combined with Nivolumab Versus Nivolumab in Participants with Metastatic or Unresectable Melanoma That Is Previously Untreated. Identifier NCT03329846. Available online: https://clinicaltrials.gov/ct2/show/NCT03329846 (accessed on 1 August 2022).
- Ning, J.T. A Multicenter, Randomized, Double-Blind Phase 3 Study of HBI-8000 Combined with Nivolumab Versus Placebo with Nivolumab in Patients with Unresectable or Metastatic Melanoma Not Previously Treated with PD-1 or PD-L1 Inhibitors. Identifier NCT04674683. Available online: https://clinicaltrials.gov/ct2/show/NCT04674683 (accessed on 1 August 2022).
- Arance, A.; de la Cruz-Merino, L.; Petrella, T.M.; Jamal, R.; Ny, L.; Carneiro, A.; Berrocal, A.; Márquez-Rodas, I.; Spreafico, A.; Atkinson, V.; et al. Phase II LEAP-004 Study of Lenvatinib Plus Pembrolizumab for Melanoma With Confirmed Progression on a Programmed Cell Death Protein-1 or Programmed Death Ligand 1 Inhibitor Given as Monotherapy or in Combination. J. Clin. Oncol. 2022, JCO2200221. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Lee, S.J.; Chmielowski, B.; Tarhini, A.A.; Cohen, G.I.; Truong, T.G.; Moon, H.H.; Davar, D.; O’Rourke, M.; Stephenson, J.J.; et al. Combination dabrafenib and trametinib versus combination nivolumab and ipilimumab for patients with advanced BRAF-mutant melanoma: The DREAMseq Trial—ECOG-ACRIN EA6134. J. Clin. Oncol. 2022, 27, 101200JCO2201763. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approvals-opdualag-nivolumab-and-relatlimab-rmbw-unresectable-or#:~:text=On%20March%2018%2C%202022%2C%20the,with%20unresectable%20or%20metastatic%20melanoma (accessed on 1 August 2022).
- Long, G.V.; Dummer, R.; Hamid, O.; Gajewski, T.F.; Caglevic, C.; Dalle, S.; Arance, A.; Carlino, M.S.; Grob, J.J.; Kim, T.M.; et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): A phase 3, randomised, double-blind study. Lancet Oncol. 2019, 20, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Lawson, D.H.; Lee, S.; Zhao, F.; Tarhini, A.A.; Margolin, K.A.; Ernstoff, M.S.; Atkins, M.B.; Cohen, G.I.; Whiteside, T.L.; Butterfield, L.H.; et al. Randomized, Placebo-Controlled, Phase III Trial of Yeast-Derived Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) Versus Peptide Vaccination Versus GM-CSF Plus Peptide Vaccination Versus Placebo in Patients with No Evidence of Disease After Complete Surgical Resection of Locally Advanced and/or Stage IV Melanoma: A Trial of the Eastern Cooperative Oncology Group-American College of Radiology Imaging Network Cancer Research Group (E4697). J. Clin. Oncol. 2015, 33, 4066–4076. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Donahue, H.; Nishino, M.; Giobbie-Hurder, A.; Davis, M.; Bailey, N.; Ott, P.A.; Hodi, F.S. Single Institution Experience of Ipilimumab 3 mg/kg with Sargramostim (GM-CSF) in Metastatic Melanoma. Cancer Immunol. Res. 2015, 3, 986–991. [Google Scholar] [CrossRef] [Green Version]
- Storkus, W.J.; Maurer, D.; Lin, Y.; Ding, F.; Bose, A.; Lowe, D.; Rose, A.; DeMark, M.; Karapetyan, L.; Taylor, J.L.; et al. Dendritic cell vaccines targeting tumor blood vessel antigens in combination with dasatinib induce therapeutic immune responses in patients with checkpoint-refractory advanced melanoma. J. Immunother. Cancer 2021, 9, e003675. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; Dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. REGARD Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014, 383, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Fruehauf, J.P.; El-Masry, M.; Osann, K.; Parmakhtiar, B.; Yamamoto, M.; Jakowatz, J.G. Phase II study of pazopanib in combination with paclitaxel in patients with metastatic melanoma. Cancer Chemother. Pharmacol. 2018, 82, 353–360. [Google Scholar] [CrossRef]
- Guo, J.; Carvajal, R.D.; Dummer, R.; Hauschild, A.; Daud, A.; Bastian, B.C.; Markovic, S.N.; Queirolo, P.; Arance, A.; Berking, C.; et al. Efficacy and safety of nilotinib in patients with KIT-mutated metastatic or inoperable melanoma: Final results from the global, single-arm, phase II TEAM trial. Ann. Oncol. 2017, 28, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Huijberts, S.; Wang, L.; de Oliveira, R.L.; Rosing, H.; Nuijen, B.; Beijnen, J.; Bernards, R.; Schellens, J.; Wilgenhof, S. Vorinostat in patients with resistant BRAFV600E mutated advanced melanoma: A proof of concept study. Future Oncol. 2020, 16, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Arozarena, I.; Wellbrock, C. Overcoming resistance to BRAF inhibitors. Ann. Transl. Med. 2017, 5, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varghese, S.; Pramanik, S.; Prasad, R.; Hodges, H.; Williams, L.; Peng, W.; Tawbi, H.; Nanda, V.Y. The glutaminase inhibitor CB-839 potentiates antimelanoma activity of standard-of-care targeted therapies and immunotherapies. In Proceedings of the AACR Special Conference on Melanoma: From Biology to Target, Houston, TX, USA, 15–18 January 2019; Volume 80. [Google Scholar]
- Varghese, S.; Pramanik, S.; Williams, L.J.; Hodges, H.R.; Hudgens, C.W.; Fischer, G.M.; Luo, C.K.; Knighton, B.; Tan, L.; Lorenzi, P.L.; et al. The Glutaminase Inhibitor CB-839 (Telaglenastat) Enhances the Antimelanoma Activity of T-Cell-Mediated Immunotherapies. Mol. Cancer Ther. 2021, 20, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Révész, L.; Edgren, M.R.; Wainson, A.A. Selective toxicity of buthionine sulfoximine (BSO) to melanoma cells in vitro and in vivo. Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Andtbacka, R.H.I.; Curti, B.; Daniels, G.A.; Hallmeyer, S.; Whitman, E.D.; Lutzky, J.; Spitler, L.E.; Zhou, K.; Bommareddy, P.K.; Grose, M.; et al. Clinical Responses of Oncolytic Coxsackievirus A21 (V937) in Patients With Unresectable Melanoma. J. Clin. Oncol. 2021, 39, 3829–3838. [Google Scholar] [CrossRef] [PubMed]
- Maciá, S. Phase 2 Single Arm Clinical Study to Evaluate the Efficacy and Safety of Intratumoral Administration of BO-112 in Combination with Pembrolizumab in Subjects That Have Progressed on Anti-PD-1-Based Therapy for Stage III or IV Melanoma. Identifier NCT04570332. Available online: https://clinicaltrials.gov/ct2/show/NCT04570332 (accessed on 1 August 2022).
- Plummer, R.; Dua, D.; Cresti, N.; Drew, Y.; Stephens, P.; Foegh, M.; Knudsen, S.; Sachdev, P.; Mistry, B.M.; Dixit, V.; et al. First-in-human study of the PARP/tankyrase inhibitor E7449 in patients with advanced solid tumours and evaluation of a novel drug-response predictor. Br. J. Cancer 2020, 123, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef]




| Type of Trial | Combination Mechanisms | Single Mechanisms |
|---|---|---|
| Late-stage Systemic |
|
|
| Late-stage Locoregional |
|
|
| Exploratory Therapies | Immunotherapy | Targeted Therapy | Chemotherapy | Metabolic Therapy | Others | Total |
|---|---|---|---|---|---|---|
| Systemic | 9 | 2 | 0 | 3 | 2 | 16 |
| Anti LAG-3 Vaccine a,d,e Cytokine a,b,c ACT Immunostimulatory T cells a | MTK Anti-VEGFR | IDO1 inhibitors HDAC inhibitors | Vitamin D Beta blockers | |||
| Locoregional | 6 | 0 | 0 | 0 | 1 | 7 |
| Cytokine f,h,k Vaccine g Immunostimulatory T cells h | PV-10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakish, H.H.; Ahmed, F.A.; Elshami, M.; Loftus, A.W.; Hoehn, R.S.; Ammori, J.B.; Ocuin, L.M.; Winter, J.M.; Bordeaux, J.S.; Mangla, A.; et al. Trends in Melanoma Phase 3 Clinical Trials since 2010: Is there Hope for Advanced Melanoma Therapies beyond Approved Treatment Mechanisms? Cancers 2022, 14, 5184. https://doi.org/10.3390/cancers14215184
Kakish HH, Ahmed FA, Elshami M, Loftus AW, Hoehn RS, Ammori JB, Ocuin LM, Winter JM, Bordeaux JS, Mangla A, et al. Trends in Melanoma Phase 3 Clinical Trials since 2010: Is there Hope for Advanced Melanoma Therapies beyond Approved Treatment Mechanisms? Cancers. 2022; 14(21):5184. https://doi.org/10.3390/cancers14215184
Chicago/Turabian StyleKakish, Hanna H., Fasih Ali Ahmed, Mohamedraed Elshami, Alexander W. Loftus, Richard S. Hoehn, John B. Ammori, Lee M. Ocuin, Jordan M. Winter, Jeremy S. Bordeaux, Ankit Mangla, and et al. 2022. "Trends in Melanoma Phase 3 Clinical Trials since 2010: Is there Hope for Advanced Melanoma Therapies beyond Approved Treatment Mechanisms?" Cancers 14, no. 21: 5184. https://doi.org/10.3390/cancers14215184