Neurointerventional Treatment of Vein of Galen Malformation (VGM): A Structured Review with a Proposal for the Comparison of Outcome Quality
Abstract
:Key Points
- Vein of Galen Malformation (VGM) is a congenital intracranial vascular anomaly consisting of arteriovenous fistulae and/or malformations.
- In neonates and infants with VGM, priority is given to the treatment of heart failure caused by excess intracranial shunt volume.
- A semiquantitative multidimensional scoring system, which allows a more objective comparison of the included studies taking into account six key dimensions, was introduced.
- The best outcome quality was found for the study “Houston” 2002–2018 (19 points) and the study “Duisburg” 2001–2010 (19 points).
- Neurointerventional treatment is the essential pillar in the interdisciplinary management of patients with VGM, although standardization is lacking—based on the results of the structured review.
- As a complementary treatment, pediatric critical care is mandatory and includes pre-, peri-, and post-neurointerventional medical hemodynamic stabilization.
- Neurosurgery and radiotherapy currently have no role as first-line treatments due to the high morbidity and mortality and/or lack of efficacy associated with the procedures.
1. Introduction
1.1. Nomenclature
1.2. Classification
1.3. Epidemiology
1.4. Clinical Symptoms and Challenges
1.5. Outcome Scores
1.6. Objectives of This Review
2. Materials and Methods
2.1. Search Strategy, Eligibility Criteria, and Data Collection
2.2. Summary Measures and Synthesis
2.3. Comparison of Outcome Quality
- Patients younger than 1 month of age at the time of neurointerventional treatment.
- Patients with severe heart failure as a dominant symptom (e.g., high-output heart failure, severe congestive heart failure, or cyanotic heart failure).
- Patients with highly complex VGM angioarchitecture (VGM Yasargil types IVa–IVc or VGM Lasjaunias type 1 “choroidal”).
- Neurointerventional treatment without procedure-related complications.
- Patients with neurologically normal or quasi-normal outcomes.
- Patients that survived.
3. Results
3.1. Summary of Measures and Synthesis
3.1.1. Study “New York” 2004–2015 [35]
3.1.2. Study “London” 2003–2008 [34]
3.1.3. Study “Houston” 2002–2018 [33]
3.1.4. Study “Duisburg” 2001–2010 [29]
3.1.5. Study “Mumbai” 1998–2012 [11]
3.1.6. Study “Philadelphia” 1994–2007 [32]
3.1.7. Study “Le Kremlin-Bicêtre” 1981–2002 [20]
3.1.8. Study “Paris” 1988–1994 [31]
3.2. Comparison of Outcome Quality
4. Discussion
4.1. Conservative Treatment
4.2. Neurosurgery
4.3. Radiotherapy
4.4. Molecular Treatment
4.5. “Practical Insights According to Institutional Standard”
4.5.1. “Pediatric Critical Care-Medical Hemodynamic Stabilization with Prostaglandin E1”
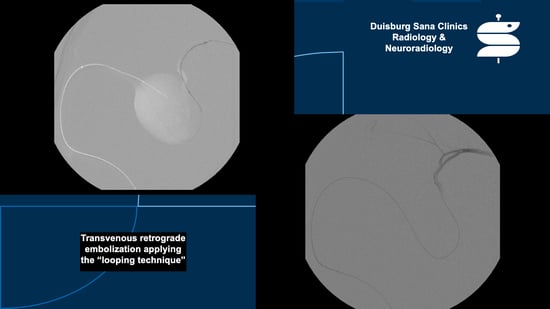
4.5.2. “Neurointerventional Treatment-Hemodynamic Stabilization with Embolization”
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brassel, F.; Meila, D.; Papke, K. Vascular interventions in the head and neck region. part 2: Procedures for vessel occlusion. Radiologe 2011, 51, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, M.M.; Griessenauer, C.J.; Foreman, P.; Bavarsad Shahripour, R.; Shoja, M.M.; Rozzelle, C.J.; Tubbs, R.S.; Fisher, W.S.; Fukushima, T. Vein of Galen Aneurysmal Malformations: Critical Analysis of the Literature with Proposal of a New Classification System. J. Neurosurg. Pediatr. 2013, 12, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Steinheil, S.O. Über einen Fall von Varix aneurysmaticusim Bereichder Gehirngefässe; Dissertationsschrift; F. Fromme: Würzburg, Germany, 1895. [Google Scholar]
- Raybaud, C.A.; Strother, C.M.; Hald, J.K. Aneurysms of the Vein of Galen: Embryonic Considerations and Anatomical Features Relating to the Pathogenesis of the Malformation. Neuroradiology 1989, 31, 109–128. [Google Scholar] [CrossRef]
- Gailloud, P.; O’riordan, D.P.; Burger, I.; Lehmann, C.U. Confirmation of Communication between Deep Venous Drainage and the Vein of Galen after Treatment of a Vein of Galen Aneurysmal Malformation in an Infant Presenting with Severe Pulmonary Hypertension. AJNR Am. J. Neuroradiol. 2006, 27, 317–320. [Google Scholar]
- Recinos, P.F.; Rahmathulla, G.; Pearl, M.; Recinos, V.R.; Jallo, G.I.; Gailloud, P.; Ahn, E.S. Vein of Galen Malformations: Epidemiology, Clinical Presentations, Management. Neurosurg. Clin. N. Am. 2012, 23, 165–177. [Google Scholar] [CrossRef]
- Khullar, D.; Andeejani, A.M.I.; Bulsara, K.R. Evolution of Treatment Options for Vein of Galen Malformations. J. Neurosurg. Pediatr. 2010, 6, 444–451. [Google Scholar] [CrossRef]
- Yaşargil, M.G.; Yaşargil, M.G. AVM of the Brain, Clinical Considerations, General and Special Operative Techniques, Surgical Results, Nonoperated Cases, Cavernous and Venous Angiomas, Neuroanesthesia; 191 Tables; Microneurosurgery; Thieme [u.a.]: Stuttgart, Germany, 1988; ISBN 978-3-13-693501-9. [Google Scholar]
- Lasjaunias, P. Vascular Diseases in Neonates, Infants and Children: Interventional Neuroradiology Management; Springer: Berlin/Heidelberg, Germany, 1997; ISBN 978-3-662-10740-9. [Google Scholar]
- Florian, I.S. Springer Nature Switzerland AG; Cham, Switzerland, 2020. [Google Scholar]
- Sivasankar, R.; Limaye, U.S.; Wuppalapati, S.; Shrivastava, M. Endovascular Management of Vein of Galen Aneurysmal Malformations: A Retrospective Analysis over a 15-Year Period. J. Vasc. Interv. Neurol. 2019, 10, 23–29. [Google Scholar] [PubMed]
- Brevis Nuñez, F.; Dohna-Schwake, C. Epidemiology, Diagnostics, and Management of Vein of Galen Malformation. Pediatr. Neurol. 2021, 119, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Jea, A.; Bradshaw, T.J.; Whitehead, W.E.; Curry, D.J.; Dauser, R.C.; Luerssen, T.G. The High Risks of Ventriculoperitoneal Shunt Procedures for Hydrocephalus Associated with Vein of Galen Malformations in Childhood: Case Report and Literature Review. Pediatr. Neurosurg. 2010, 46, 141–145. [Google Scholar] [CrossRef]
- Hassan, T.; Nassar, M.; Elghandour, M. Vein of Galen Aneurysms: Presentation and Endovascular Management. Pediatr. Neurosurg. 2010, 46, 427–434. [Google Scholar] [CrossRef]
- Berenstein, A.; Fifi, J.T.; Niimi, Y.; Presti, S.; Ortiz, R.; Ghatan, S.; Rosenn, B.; Sorscher, M.; Molofsky, W. Vein of Galen Malformations in Neonates: New Management Paradigms for Improving Outcomes. Neurosurgery 2012, 70, 1207–1213, discussion 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- Howarth, R.A.; Reisner, A.; Chern, J.J.; Hayes, L.L.; Burns, T.G.; Berenstein, A. Neurocognitive Improvements Following Endovascular Repair of Vein of Galen Malformation in a Child. J. Neurosurg. Pediatr. 2015, 15, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Hoang, S.; Choudhri, O.; Edwards, M.; Guzman, R. Vein of Galen Malformation. Neurosurg. Focus 2009, 27, E8. [Google Scholar] [CrossRef]
- Chow, M.L.; Cooke, D.L.; Fullerton, H.J.; Amans, M.R.; Narvid, J.; Dowd, C.F.; Higashida, R.T.; Halbach, V.V.; Hetts, S.W. Radiological and Clinical Features of Vein of Galen Malformations. J. Neurointerv. Surg. 2015, 7, 443–448. [Google Scholar] [CrossRef]
- Brinjikji, W.; Krings, T.; Murad, M.H.; Rouchaud, A.; Meila, D. Endovascular Treatment of Vein of Galen Malformations: A Systematic Review and Meta-Analysis. AJNR Am. J. Neuroradiol. 2017, 38, 2308–2314. [Google Scholar] [CrossRef] [PubMed]
- Lasjaunias, P.L.; Chng, S.M.; Sachet, M.; Alvarez, H.; Rodesch, G.; Garcia-Monaco, R. The Management of Vein of Galen Aneurysmal Malformations. Neurosurgery 2006, 59, S184–S194, discussion S3–S13. [Google Scholar] [CrossRef]
- Jones, B.V.; Ball, W.S.; Tomsick, T.A.; Millard, J.; Crone, K.R. Vein of Galen Aneurysmal Malformation: Diagnosis and Treatment of 13 Children with Extended Clinical Follow-Up. AJNR Am. J. Neuroradiol. 2002, 23, 1717–1724. [Google Scholar]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Sommer, C.M.; Pieper, C.C.; Offensperger, F.; Pan, F.; Killguss, H.J.; Köninger, J.; Loos, M.; Hackert, T.; Wortmann, M.; Do, T.D.; et al. Radiological Management of Postoperative Lymphorrhea. Langenbecks Arch. Surg. 2021, 406, 945–969. [Google Scholar] [CrossRef]
- Alektoroff, K.; Papanagiotou, P. Vein of Galen aneurysmal malformation. Die Radiologie 2022, 62, 671–674. [Google Scholar] [CrossRef]
- Reddy, R.; Lucke-Wold, B. Primer of Vein of Galen Malformation Management. J. Pediatr. Heath Care Med. 2022, 5, 30–34. [Google Scholar]
- Cory, M.J.; Durand, P.; Sillero, R.; Morin, L.; Savani, R.; Chalak, L.; Angelis, D. Vein of Galen Aneurysmal Malformation: Rationalizing Medical Management of Neonatal Heart Failure. Pediatr. Res. 2022, 93, 39–48. [Google Scholar] [CrossRef] [PubMed]
- See, A.P.; Wilkins-Haug, L.E.; Benson, C.B.; Tworetzky, W.; Orbach, D.B. Percutaneous Transuterine Fetal Cerebral Embolisation to Treat Vein of Galen Malformations at Risk of Urgent Neonatal Decompensation: Study Protocol for a Clinical Trial of Safety and Feasibility. BMJ Open 2022, 12, e058147. [Google Scholar] [CrossRef] [PubMed]
- Fifi, J.T.; Bazil, M.J.; Matsoukas, S.; Shigematsu, T.; Sorscher, M.; Berenstein, A. Evolution of Transvenous Embolization in Vein of Galen Malformation: Case Series and Review of the Literature. J. Neurointerv. Surg. 2022, 14, neurintsurg-2022-019121. [Google Scholar] [CrossRef] [PubMed]
- Meila, D.; Hannak, R.; Feldkamp, A.; Schlunz-Hendann, M.; Mangold, A.; Jacobs, C.; Papke, K.; Brassel, F. Vein of Galen Aneurysmal Malformation: Combined Transvenous and Transarterial Method Using a “Kissing Microcatheter Technique”. Neuroradiology 2012, 54, 51–59. [Google Scholar] [CrossRef]
- Lasjaunias, P.; Berenstein, A.; Ter Brugge, K.G. Surgical Neuroangiography: Vol. 3: Clinical and Interventional Aspects in Children, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-41681-4. [Google Scholar]
- Borthne, A.; Carteret, M.; Baraton, J.; Courtel, J.; Brunelle, F. Vein of Galen Vascular Malformations in Infants: Clinical, Radiological and Therapeutic Aspect. Eur. Radiol. 1997, 7, 1252–1258. [Google Scholar] [CrossRef]
- Heuer, G.G.; Gabel, B.; Beslow, L.A.; Stiefel, M.F.; Schwartz, E.S.; Storm, P.B.; Ichord, R.N.; Hurst, R.W. Diagnosis and Treatment of Vein of Galen Aneurysmal Malformations. Childs Nerv. Syst. 2010, 26, 879–887. [Google Scholar] [CrossRef]
- Wagner, K.M.; Ghali, M.G.Z.; Srinivasan, V.M.; Lam, S.; Johnson, J.; Chen, S.; Kan, P. Vein of Galen Malformations: The Texas Children’s Hospital Experience in the Modern Endovascular Era. Oper. Neurosurg. 2019, 17, 286–292. [Google Scholar] [CrossRef]
- McSweeney, N.; Brew, S.; Bhate, S.; Cox, T.; Roebuck, D.J.; Ganesan, V. Management and Outcome of Vein of Galen Malformation. Arch. Dis. Child. 2010, 95, 903–909. [Google Scholar] [CrossRef]
- Berenstein, A.; Paramasivam, S.; Sorscher, M.; Molofsky, W.; Meila, D.; Ghatan, S. Vein of Galen Aneurysmal Malformation: Advances in Management and Endovascular Treatment. Neurosurgery 2019, 84, 469–478. [Google Scholar] [CrossRef]
- Winn, H.R.; Youmans, J.R. (Eds.) Youmans Neurological Surgery, 5th ed.; W.B. Saunders: Philadelphia, PA, USA, 2004; ISBN 978-0-7216-8291-4. [Google Scholar]
- Hansen, D.; Kan, P.T.; Reddy, G.D.; Mohan, A.C.; Jea, A.; Lam, S. Pediatric Knowledge Update: Approach to the Management of Vein of Galen Aneurysmal Malformations in Neonates. Surg. Neurol. Int. 2016, 7, S317–S321. [Google Scholar] [CrossRef]
- Fullerton, H.J.; Aminoff, A.R.; Ferriero, D.M.; Gupta, N.; Dowd, C.F. Neurodevelopmental Outcome after Endovascular Treatment of Vein of Galen Malformations. Neurology 2003, 61, 1386–1390. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.H.; Whittle, I.R.; Besser, M.; Morgan, M.K. Vein of Galen Malformation: Diagnosis and Management. Neurosurgery 1987, 20, 747–758. [Google Scholar] [CrossRef]
- Norman, M.G.; Becker, L.E. Cerebral Damage in Neonates Resulting from Arteriovenous Malformation of the Vein of Galen. J. Neurol. Neurosurg. Psychiatry 1974, 37, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.J.; Chuang, S.; Hendrick, E.B.; Humphreys, R.P. Aneurysms of the Vein of Galen. Experience at the Hospital for Sick Children, Toronto. J. Neurosurg. 1982, 57, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Ciricillo, S.F.; Edwards, M.S.; Schmidt, K.G.; Hieshima, G.B.; Silverman, N.H.; Higashida, R.T.; Halbach, V.V. Interventional Neuroradiological Management of Vein of Galen Malformations in the Neonate. Neurosurgery 1990, 27, 22–27, discussion 27–28. [Google Scholar] [CrossRef]
- Gupta, A.K.; Rao, V.R.K.; Varma, D.R.; Kapilamoorthy, T.R.; Kesavadas, C.; Krishnamoorthy, T.; Thomas, B.; Bodhey, N.K.; Purkayastha, S. Evaluation, Management, and Long-Term Follow up of Vein of Galen Malformations. J. Neurosurg. 2006, 105, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Payne, B.R.; Prasad, D.; Steiner, M.; Bunge, H.; Steiner, L. Gamma Surgery for Vein of Galen Malformations. J. Neurosurg. 2000, 93, 229–236. [Google Scholar] [CrossRef]
- Triffo, W.J.; Bourland, J.D.; Couture, D.E.; McMullen, K.P.; Tatter, S.B.; Morris, P.P. Definitive Treatment of Vein of Galen Aneurysmal Malformation with Stereotactic Radiosurgery. J. Neurosurg. 2014, 120, 120–125. [Google Scholar] [CrossRef]
- Watban, J.A.; Rodesch, G.; Alvarez, H.; Lasjaunias, P. Transarterial Embolization of Vein of Galen Aneurysmal Malformation after Unsuccessful Stereotactic Radiosurgery. Report of Three Cases. Childs Nerv. Syst. 1995, 11, 406–408. [Google Scholar] [CrossRef]
- Choque-Velasquez, J.; Colasanti, R.; Muhammad, S.; Chioffi, F.; Hernesniemi, J. Vascular Lesions of the Pineal Region: A Comprehensive Review of the Therapeutic Options. World Neurosurg. 2022, 159, 298–313. [Google Scholar] [CrossRef]
- Duran, D.; Karschnia, P.; Gaillard, J.R.; Karimy, J.K.; Youngblood, M.W.; DiLuna, M.L.; Matouk, C.C.; Aagaard-Kienitz, B.; Smith, E.R.; Orbach, D.B.; et al. Human Genetics and Molecular Mechanisms of Vein of Galen Malformation. J. Neurosurg. Pediatr. 2018, 21, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Goumans, M.-J.; Liu, Z.; ten Dijke, P. TGF-Beta Signaling in Vascular Biology and Dysfunction. Cell Res. 2009, 19, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, L.; Durand, P.; Morin, L.; Miatello, J.; Merchaoui, Z.; Lambert, V.; Boithias, C.; Senat, M.V.; Stos, B.; Maurey, H.; et al. Management and Outcomes of Neonatal Arteriovenous Brain Malformations with Cardiac Failure: A 17 Years’ Experience in a Tertiary Referral Center. J. Pediatr. 2020, 218, 85–91.e2. [Google Scholar] [CrossRef] [PubMed]
- Giesinger, R.E.; Elsayed, Y.N.; Castaldo, M.P.; McNamara, P.J. Targeted Neonatal Echocardiography-Guided Therapy in Vein of Galen Aneurysmal Malformation: A Report of Two Cases with a Review of Physiology and Approach to Management. AJP Rep. 2019, 9, e172–e176. [Google Scholar] [CrossRef] [PubMed]
- Brassel, F.; Kobba, S.; Sommer, C.M. Vein of Galen Aneurysmal Malformation (VGAM); Chapter 23 in Region Lesions; Springer Nature Switzerland AG: Cham, Switzerland, 2020. [Google Scholar]
- Brassel, F. Lecture: Approach—Vein of Galen Malformations; World AVM Congress 2022; Val d’Isère, France, 2022. [Google Scholar]





| Type | Arterial Feeders | Venous Outflow |
|---|---|---|
| I (arteriovenous fistula only) | pericallosal artery and P3 segment of the posterior cerebral artery | internal cerebral veins and atrial veins |
| II (arteriovenous fistula only) | P1 segment of the posterior cerebral artery | dilated MPV and (potentially) internal cerebral veins and atrial veins |
| III (arteriovenous fistula only) | Pericallosal artery, P3 segment of the posterior cerebral artery, P1 segment of the posterior cerebral artery, and thalamoperforating arteries | dilated MPV and (potentially) internal cerebral veins and atrial veins |
| IVa (thalamic arteriovenous malformation) | P1 segment of the posterior cerebral artery and thalamoperforating arteries | dilated MPV |
| IVb (mesencephalic arteriovenous malformation) | posterior communicating artery, P1 segment of the posterior cerebral artery, and P3 segment of the posterior cerebral artery | dilated MPV and (potentially) internal cerebral veins and atrial veins |
| IVc (mesodiencephalic arteriovenous malformation and arteriovenous fistula) | posterior communicating artery and P1 segment of the posterior cerebral artery (malformation component) pericallosal artery and P3 segment of the posterior cerebral artery (fistula component) | dilated MPV |
| Type | Arterial Feeders | Venous Outflow |
|---|---|---|
| 1 “Choroidal” | anterior choroidal artery, posterior choroidal artery, anterior cerebral artery, thalamoperforating arteries, and/or collicular and quadrigeminal arteries | ventral portion of the MPV |
| 2 “Mural” | collicular or quadrigeminal artery and/or posterior choroidal artery | inferior and lateral portions of the MPV |
| Scoring | Heart Function | Brain Function | Lung Function | Liver Function | Kidney Function |
|---|---|---|---|---|---|
| 5 Points | normal | normal | normal | - | - |
| 4 Points | overload, no medical treatment | subclinical isolated EEG abnormalities | tachypnea, does finish bottle | - | - |
| 3 Points | failure; stable with medical treatment | non-convulsive treatment, neurologic signs | tachypnea, does not finish bottle | no hepatomegaly, normal hepatic functions | normal |
| 2 Points | failure; unstable with medical treatment | isolated convulsion | assisted ventilation, normal saturation IO2F < 25% | Hepatomegaly, Normal hepatic functions | transient anuria |
| 1 Point | assisted ventilation necessary | seizures | assisted ventilation, normal saturation IO2F > 25% | moderate or transient hepatic insufficiency | unstable diuresis with treatment |
| 0 Points | refractory to medical treatment | permanent neurological signs | assisted ventilation, desaturation | abnormal coagulation, elevated enzymes | anuria |
| Study | Proportion of Patients Undergoing Neurointerventional Treatment | Age of Patients (Percentage of Patients Younger than 1 Month of Age at the Time of Neurointerventional Treatment *) | Clinical Indications (Percentage of Patients with Severe Heart Failure as a Dominant Symptom) |
|---|---|---|---|
| Study “New York” 2004–2015 | 45/45 | 1 month–>5 years (0%) | heart failure; pulmonary arterial hypertension; macrocephalus; hydrocephalus; headache; pulsatile dilated facial veins; cognitive decline; seizures (0%) |
| Study “London” 2003–2008 | 28/33 | 1 day–18 months (n.s.) | heart failure; macrocephalus; seizures; vomiting; unnatural gait (58%) |
| Study “Houston” 2002–2018 | 16/18 | n.s. (67%) | heart failure; seizures; motor deficits; dilated scalp veins; hydrocephalus; headache/nausea (50%) |
| Study “Duisburg” 2001–2010 | 14/14 | 1 day–17 months (57%) | heart failure; macrocephalus; hydrocephalus; seizures; cerebral ischemia (57%) |
| Study “Mumbai” 1998–2012 | 26/26 | 1 day–18 years (4%) | heart failure/dyspnea on feeding; macrocephalus; developmental delay of neurocognitive functioning; seizures; failure to thrive; dilated scalp veins; visual disturbances; focal neurological deficits; headache (4%) |
| Study “Philadelphia” 1994–2007 | 11/13 | 1 day–31 months (55%) | heart failure; seizures; cerebral ischemia; intracranial hemorrhage; leukomalacia; cerebral atrophy (45%) |
| Study “Le Kremlin-Bicêtre” 1981–2002 | 216 1/317 | <1 month–16 years 1 (38% 1) | heart failure; macrocephalus; hydrocephalus; seizures; mental retardation; cerebral atrophy; sinus thrombosis; pial reflux 1 (n.s. 1) |
| Study “Paris” 1988–1994 | 13/14 | 1 month–5.5 years (38%) | heart failure; hydrocephalus; streaming skull murmur; dilated facial veins; cerebral atrophy; visual disturbances (nystagmus, strabismus, papilledema); seizures; developmental delay (21%) |
| Study | VGM Angioarchitecture (Classification) | Treatment Technique (Embolic Material) | Degree of VGM Obliteration * | Procedure-Related Complication Rate |
|---|---|---|---|---|
| Study “New York” 2004–2015 | highly complex: 73% 1 (VGM Lasjaunias type 1 “choroidal”) | transarterial and/or transvenous (glue/iodized oil, tantalum, EVOH, coils in exceptional cases) | complete: 82%, partial: 13% n.s.: 4% | 11% 2 |
| Study “London” 2003–2008 | highly complex: 61% 1 (VGM Lasjaunias type 1 “choroidal”) | transarterial (glue, presumably together with iodized oil) | complete: 39%, partial: 54% n.s.: 7% | 43% 2 |
| Study “Houston” 2002–2018 | highly complex: 78% 1 (VGM Lasjaunias type 1 “choroidal”) | transarterial (glue, presumably together with iodized oil, EVOH, coils, balloon) | complete: 20%, partial: 80% | 24% 3 |
| Study “Duisburg” 2001–2010 | highly complex: 86% 1 (VGM Lasjaunias type 1 “choroidal”) | combined transarterial and transvenous (coils) | complete: 57% > 90%: 21% 50%: 21% | 9% 3 |
| Study “Mumbai” 1998–2012 | highly complex: 42% 1 (VGM Lasjaunias type 1 “choroidal”) | transarterial (glue/tantalum mixture, glue/iodized oil mixture, EVOH) | n.s. | 31%2 |
| Study “Philadelphia” 1994–2007 | highly complex: 62% 1 (VGM Lasjaunias type 1 “choroidal”) | transarterial (glue, presumably together with iodized oil, coils) | n.s. | 36% 2 |
| Study “Le Kremlin-Bicêtre” 1981–2002 | n.s. 3 | transarterial and/or transvenous 3 (glue/tantalum/iodized oil mixture3) | >90%: 55% 4 50–90%: 39% 4 <50%: 6% 4 | 17% 2,4 |
| Study “Paris” 1988–1994 | highly complex: 0% 1 (VGM Yasargil types I–III) | transarterial or transvenous (glue/iodized oil mixture, nylon filament, coils) | n.s. | n.s. |
| Study | Neurologically Normal or Quasi-Normal Neurologically Moderately Impaired Neurologically Severely Impaired Dead | Overall Survival Rate | Follow-Up Period |
|---|---|---|---|
| Study “New York” 2004–2015 | 87% 9% 0% 4% | 96% 1 | n.s. |
| Study “London” 2003–2008 | 61% 1 0% 1 18% 1 7% 1 | 79% 1 | mean of 33 months |
| Study “Houston” 2002–2018 | 67% 1 n.s. 1 17% 1 6% 1 | 94% 1 | mean of 38 months |
| Study “Duisburg” 2001–2010 | 64% 14% 14% 7% | 93% 1 | mean of 53 months |
| Study “Mumbai” 1998–2012 | 85% 0% 4% 12% | 88% | n.s. |
| Study “Philadelphia” 1994–2007 | 54% 1 8% 1 8% 1 15% 1 | 77% 1 | mean of 50 months |
| Study “Le Kremlin-Bicêtre” 1981–2002 | 66% 2,3 14% 2,3 9% 2,3 11% 2,3 | 89% 2,3 | n.s. |
| Study “Paris” 1988–1994 | n.s n.s n.s. 29% | 71% 1 | n.s. |
| Study | 1 1 | 2 2 | 3 3 | 4 4 | 5 5 | 6 6 | Total Score | Final Study Ranking |
|---|---|---|---|---|---|---|---|---|
| Study “New York” 2004–2015 | 1 | 1 | 3 | 3 | 4 | 4 | 16 | sole #3 |
| Study “London” 2003–2008 | 0 | 4 | 2 | 1 | 1 | 2 | 10 | shared #4 |
| Study “Houston” 2002–2018 | 4 | 3 | 3 | 2 | 3 | 4 | 19 | shared #1 |
| Study “Duisburg” 2001–2010 | 3 | 3 | 4 | 4 | 2 | 3 | 19 | shared #1 |
| Study “Mumbai” 1998–2012 | 1 | 1 | 1 | 2 | 3 | 2 | 10 | shared #4 |
| Study “Philadelphia” 1994–2007 | 3 | 2 | 2 | 1 | 1 | 1 | 10 | shared #4 |
| Study “Le Kremlin-Bicêtre” 1981–2002 | 2 | 0 | 0 | 3 | 2 | 3 | 10 | shared #4 |
| Study “Paris” 1988–1994 | 2 | 2 | 1 | 0 | 0 | 1 | 6 | sole #8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brassel, F.; Schlunz-Hendann, M.; Scholz, M.; Lucaciu, R.; Fan, C.; Koch, V.; Grieb, D.; Brevis Nunez, F.; Schwarz, S.; Sommer, C.M. Neurointerventional Treatment of Vein of Galen Malformation (VGM): A Structured Review with a Proposal for the Comparison of Outcome Quality. J. Vasc. Dis. 2023, 2, 236-258. https://doi.org/10.3390/jvd2020018
Brassel F, Schlunz-Hendann M, Scholz M, Lucaciu R, Fan C, Koch V, Grieb D, Brevis Nunez F, Schwarz S, Sommer CM. Neurointerventional Treatment of Vein of Galen Malformation (VGM): A Structured Review with a Proposal for the Comparison of Outcome Quality. Journal of Vascular Diseases. 2023; 2(2):236-258. https://doi.org/10.3390/jvd2020018
Chicago/Turabian StyleBrassel, Friedhelm, Martin Schlunz-Hendann, Martin Scholz, Robert Lucaciu, Chunfu Fan, Vitali Koch, Dominik Grieb, Francisco Brevis Nunez, Simone Schwarz, and Christof M. Sommer. 2023. "Neurointerventional Treatment of Vein of Galen Malformation (VGM): A Structured Review with a Proposal for the Comparison of Outcome Quality" Journal of Vascular Diseases 2, no. 2: 236-258. https://doi.org/10.3390/jvd2020018