Diagnostic and Prognostic Value of Quantitative Computed Tomography Parameters of Adrenal Glands in Patients from Internistled ICU with Sepsis and Septic Shock
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. CT–Data Acquisition
2.3. Clinical Parameters
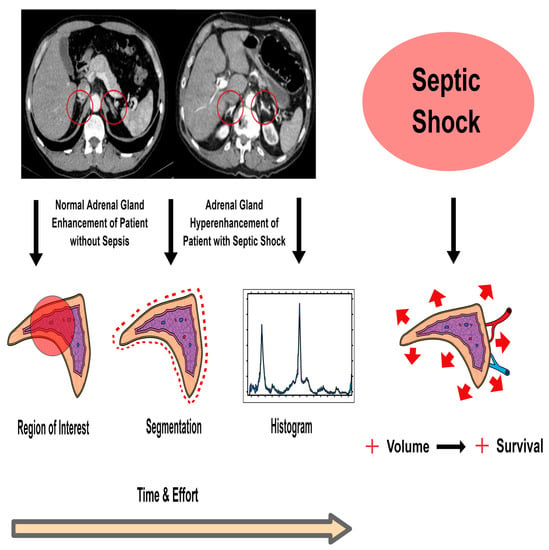
2.4. CT–Image Analysis
2.5. Statistical Analysis
3. Results
3.1. Final Cohort
3.2. Correlation of HU Attenuation Values for Right and Left Adrenal Gland
3.3. Method of Region of Interest (ROI)
3.4. Method of Semi-Automated Organ Segmentation
3.5. Histogram Analysis
3.6. Comparison of Image Analysis Methods
3.6.1. Region of Interest
3.6.2. Segmentation
3.6.3. Histogram
4. Discussion
4.1. Hyperattenuating Adrenal Glands and Sepsis
4.2. Adrenal Gland Volume and Septic Shock
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Methods | Groups | No Sepsis (1) | Sepsis (2) | Septic Shock (3) | |||
|---|---|---|---|---|---|---|---|
| n = 6 | n = 16 (15) | n = 22 (21) | |||||
| Region of Interest (ROI) | mean | 90 | 14 * | 71 | −10 * | 96 | 5 * |
| median | 97 | 24 * | 72.0 | −9 * | 90 | 2 * | |
| IQR | 26 | 23 * | 23 | 17 * | 39 | 39 * | |
| p-value 1 < 0.05 | p = 0.023 | ||||||
| Segmentation | mean | 72 | 12 * | 49 | −10 * | 71 | 5 * |
| median | 76 | 15 * | 49 | −11 * | 69 | 5 * | |
| IQR | 25 | 24 * | 32 | 21 * | 29 | 23 * | |
| p-value 1 < 0.05 | p = 0.013/0.048 * | ||||||
| Histogram | mean | 101 | 6 * | 90 | −6 * | 105 | 3 * |
| median | 106 | 12 * | 91 | −6 * | 102 | 3 * | |
| IQR | 21 | 21 * | 21 | 13 * | 25 | 3 * | |
| p-value 1 < 0.05 | p = 0.042 | p = 0.010 | |||||
References
- Engel, C.; Brunkhorst, F.M.; Bone, H.-G.; Brunkhorst, R.; Gerlach, H.; Grond, S.; Gründling, M.; Huhle, G.; Jaschinski, U.; John, S.; et al. Epidemiology of sepsis in Germany: Results from a national prospective multicenter study. Intensive Care Med. 2007, 33, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Sepsis und MODS; Springer: Berlin/Heidelberg, Germany, 2016.
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef] [PubMed]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M.; for the Sepsis Definitions Task Force. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Lindner, H.A.; Balaban, Ü.; Sturm, T.; Symbol, C.W.B.; Thiel, M.; Schneider-Lindner, V. An Algorithm for Systemic Inflammatory Response Syndrome Criteria–Based Prediction of Sepsis in a Polytrauma Cohort. Crit. Care Med. 2016, 44, 2199–2207. [Google Scholar] [CrossRef]
- Vincent, J.L.; De Mendonça, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.; Sprung, C.; FCCM; Colardyn, F.; Blecher, S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef]
- Schlegel, N.; Flemming, S.; Meir, M.; Germer, C.T. Is a different view on the pathophysiology of sepsis the key for novel therapeutic options? Chirurg 2014, 85, 714–719. [Google Scholar] [CrossRef]
- O’Hara, S.M.; Donnelly, L.F. Intense contrast enhancement of the adrenal glands: Another abdominal CT finding associated with hypoperfusion complex in children. Am. J. Roentgenol. 1999, 173, 995–997. [Google Scholar] [CrossRef] [Green Version]
- Schek, J.; Macht, S.; Klasen-Sansone, J.; Heusch, P.; Kröpil, P.; Witte, I.; Antoch, G.; Lanzman, R.S. Clinical impact of hyperattenuation of adrenal glands on contrast-enhanced computed tomography of polytraumatised patients. Eur. Radiol. 2014, 24, 527–530. [Google Scholar] [CrossRef]
- Bollen, T.L.; Dutch Acute Pancreatitis Study Group; Van Santvoort, H.C.; Besselink, M.G.H.; Van Ramshorst, B.; Van Es, H.W.; Gooszen, H.G. Intense adrenal enhancement in patients with acute pancreatitis and early organ failure. Emerg. Radiol. 2007, 14, 317–322. [Google Scholar] [CrossRef]
- Peng, Y.; Xie, Q.; Wang, H.; Lin, Z.; Zhang, F.; Zhou, X.; Guan, J. The hollow adrenal gland sign: A newly described enhancing pattern of the adrenal gland on dual-phase contrast-enhanced CT for predicting the prognosis of patients with septic shock. Eur. Radiol. 2019, 29, 5378–5385. [Google Scholar] [CrossRef] [PubMed]
- Kanczkowski, W.; Sue, M.; Zacharowski, K.; Reincke, M.; Bornstein, S.R. The role of adrenal gland microenvironment in the HPA axis function and dysfunction during sepsis. Mol. Cell. Endocrinol. 2015, 408, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.E.; Feng, Y.; Velazquez, H.; Sessa, W.C. Endothelial glucocorticoid receptor is required for protection against sepsis. Proc. Natl. Acad. Sci. USA 2013, 110, 306–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turrin, N.P.; Rivest, S. Unraveling the Molecular Details Involved in the Intimate Link between the Immune and Neuroendocrine Systems. Exp. Biol. Med. 2004, 229, 996–1006. [Google Scholar] [CrossRef]
- Boonen, E.; Bornstein, S.R.; Berghe, G.V.D. New insights into the controversy of adrenal function during critical illness. Lancet Diabetes Endocrinol. 2015, 3, 805–815. [Google Scholar] [CrossRef]
- Boonen, E.; Berghe, G.V.D. Understanding the HPA response to critical illness: Novel insights with clinical implications. Intensive Care Med. 2015, 41, 131–133. [Google Scholar] [CrossRef]
- Chanques, G.; Annane, D.; Jaber, S.; Gallix, B. Enlarged adrenals during septic shock. Intensive Care Med. 2007, 33, 1671–1672. [Google Scholar] [CrossRef]
- Prasad, K.R.; Kumar, A.; Gamanagatti, S.; Chandrashekhara, S.H. CT in post-traumatic hypoperfusion complex—A pictorial review. Emerg. Radiol. 2010, 18, 139–143. [Google Scholar] [CrossRef]
- Bae, K.T.; Fuangtharnthip, P.; Prasad, S.R.; Joe, B.N.; Heiken, J.P. Adrenal Masses: CT Characterization with Histogram Analysis Method. Radiology 2003, 228, 735–742. [Google Scholar] [CrossRef] [Green Version]
- Halefoglu, A.M.; Yasar, A.; Bas, N.; Ozel, A.; Erturk, Ş.M.; Basak, M. Comparison of computed tomography histogram analysis and chemical-shift magnetic resonance imaging for adrenal mass characterization. Acta Radiol. 2009, 50, 1071–1079. [Google Scholar] [CrossRef]
- Spiriev, T.; Nakov, V.; Laleva, L.; Tzekov, C. OsiriX software as a preoperative planning tool in cranial neurosurgery: A step-by-step guide for neurosurgical residents. Surg. Neurol. Int. 2017, 8, 241. [Google Scholar] [CrossRef] [PubMed]
- Wolf, I.; Vetter, M.; Wegner, I.; Nolden, M.; Bottger, T.; Hastenteufel, M.; Schobinger, M.; Kunert, T.; Meinzer, H.P. The medical imaging interaction toolkit (MITK): A toolkit facilitating the creation of interactive software by extending VTK and ITK. In Medical Imaging 2004: Visualization, Image-Guided Procedures, and Display; International Society for Optics and Pho-tonics: 2004; SPIE: San Diego, CA, USA, 2014; Volume 5367, pp. 16–27. [Google Scholar]
- Wang, X.; Jin, Z.-Y.; Xue, H.-D.; Liu, W.; Sun, H.; Chen, Y.; Xu, K. Evaluation of Normal Adrenal Gland Volume by 64-slice CT. Chin. Med. Sci. J. 2012, 27, 220–224. [Google Scholar] [CrossRef]
- Rotondo, A.; Angelelli, G.; Catalano, O.; Grassi, R.; Scialpi, M.; Stellacci, G.; Derchi, L.E. Abdominal computed tomographic findings in adults with hypovolemic shock. Emerg. Radiol. 1997, 4, 10–15. [Google Scholar] [CrossRef]
- Cheung, S.; Lee, R.; Tung, H.; Chan, F. Persistent Adrenal Enhancement may be the Earliest CT Sign of Significant Hypovolaemic Shock. Clin. Radiol. 2003, 58, 315–318. [Google Scholar] [CrossRef]
- Jung, B.; Nougaret, S.; Chanques, G.; Mercier, G.; Cisse, M.; Aufort, S.; Gallix, B.; Annane, D.; Jaber, S. The absence of adrenal gland enlargement during septic shock predicts mortality: A computed tomography study of 239 patients. Anesthesiology 2011, 115, 334–343. [Google Scholar] [CrossRef] [Green Version]
- Nougaret, S.; Jung, B.; Aufort, S.; Chanques, G.; Jaber, S.; Gallix, B. Adrenal gland volume measurement in septic shock and control patients: A pilot study. Eur. Radiol. 2010, 20, 2348–2357. [Google Scholar] [CrossRef]
- Mongardon, N.; Savary, G.; Geri, G.; El Bejjani, M.-R.; Silvera, S.; Dumas, F.; Charpentier, J.; Pène, F.; Mira, J.-P.; Cariou, A. Prognostic value of adrenal gland volume after cardiac arrest: Association of CT-scan evaluation with shock and mortality. Resuscitation 2018, 129, 135–140. [Google Scholar] [CrossRef]
- Adrie, C.; Adib-Conquy, M.; Laurent, I.; Monchi, M.; Vinsonneau, C.; Fitting, C.; Fraisse, F.; Dinh-Xuan, A.T.; Carli, P.; Spaulding, C.; et al. Successful Cardiopulmonary Resuscitation After Cardiac Arrest as a “Sepsis-Like” Syndrome. Circulation 2002, 106, 562–568. [Google Scholar] [CrossRef]
- Venkatanarasimha, N.; Roobottom, C. Intense Adrenal Enhancement: A Feature of Hypoperfusion Complex. Am. J. Roentgenol. 2010, 195, W82. [Google Scholar] [CrossRef]
- Johnson, P.T.; Horton, K.M.; Fishman, E.K. Adrenal Imaging with Multidetector CT: Evidence-based Protocol Optimization and Interpretative Practice. Radiographics 2009, 29, 1319–1331. [Google Scholar] [CrossRef] [Green Version]
- Taner, A.T.; Schieda, N.; Siegelman, E.S. Pitfalls in Adrenal Imaging. Semin. Roentgenol. 2015, 50, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Freel, E.M.; Nicholas, R.S.; Sudarshan, T.; Priba, L.; Gandy, S.J.; McMillan, N.; Houston, J.G.; Connell, J.M. Assessment of the accuracy and reproducibility of adrenal volume measurements using MRI and its relationship with corticosteroid phenotype: A normal volunteer pilot study. Clin. Endocrinol. 2013, 79, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Grant, L.A.; Napolitano, A.; Miller, S.; Stephens, K.; McHugh, S.M.; Dixon, A.K. A pilot study to assess the feasibility of measurement of adrenal gland volume by magnetic resonance imaging. Acta Radiol. 2010, 51, 117–120. [Google Scholar] [CrossRef]
- Amsterdam, J.D.; Marinelli, D.L.; Arger, P.; Winokur, A. Assessment of adrenal gland volume by computed tomography in depressed patients and healthy volunteers: A pilot study. Psychiatry Res. 1987, 21, 189–197. [Google Scholar] [CrossRef]
- Pojunas, K.; Daniels, D.; Williams, A.; Thorsen, M.; Haughton, V. Pituitary and adrenal CT of Cushing syndrome. Am. J. Roentgenol. 1986, 146, 1235–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| No Sepsis (n = 6) | Sepsis (n = 16) | Septic Shock (n = 22) | |
|---|---|---|---|
| Age, years (mean, range) | 74 (33 to 89) | 67 (19 to 87) | 63 (15 to 82) |
| Gender, n (%) | |||
| Male | 3 (50) | 11 (69) | 10 (46) |
| Female | 3 (50) | 5 (31) | 12 (54) |
| Site of infection, n (%) | |||
| Lung | 4 (67) | 10 (63) | 15 (68) |
| Abdominal | 1 (33) | 2 (13) | 2 |
| Urinary tract | - | 1 (6) | 1 |
| Skin | - | - | 1 |
| Heart | - | 1 (6) | 1 |
| Neck | - | 1 (6) | - |
| Blood | - | - | - |
| Others | - | 1 (6) | - |
| Laboratory values, mean ± SEM | |||
| White blood cells, 109/L | 11.0 ± 1.6 | 11.5 ± 1.7 | 18.1 ± 3.2 |
| Platelets, 109/L | 175.2 ± 39.8 | 175.6 ± 26.4 | 199.1 ± 30.2 |
| Creatinine, mg/dL | 1.40 ± 0.3 | 2.0 ± 0.4 | 8.4 ± 6.6 |
| C-reactive protein, mg/L | 152.2 ± 30.2 | 188.4 ± 28.5 | 153.8 ± 29.8 |
| pCO2, mmHg | 34.1 ± 6.1 | 44.3 ± 2.3 | 43.9 ± 2.8 |
| Lactate, mmol/L | 1.4 ± 0.2 | 1.3 ± 0.2 | 4.5 ± 0.4 |
| Positive blood cultures, n (%) | 2 (33) | 10 (63) | 9 (41) |
| ICU parameters, mean ± SEM | |||
| ICU days | 15 ± 6.5 | 20 ± 4 | 13 ± 3 |
| Ventilation days | 13 ± 6.4 | 10 ± 4 | 11 ± 3 |
| Catecholamine days | 11 ± 5.6 | 11 ± 3 | 10 ± 3 |
| Antibiotic treatment days | 12 ± 4.3 | 17 ± 4 | 12 ± 3 |
| Renal replacement therapy days | 5 ± 4.1 | 2 ± 1 | 3 ± 1 |
| GCS | 12 ± 2 | 7 ± 1 | 6 ± 1 |
| APACHE II, mean ± SEM | 18 ± 4 | 22 ± 2 | 28 ± 1 |
| SAPS II, mean ± SEM | 36 ± 6 | 40 ± 5 | 49 ± 3 |
| SOFA score, mean ± SEM | 7 ± 2 | 9 ± 1 | 12 ± 1 |
| All-cause mortality, n (%) | |||
| 8 days | |||
| Death | 0 (0) | 4 (25) | 10 (46) |
| Survivor | 6 (100) | 12 (75) | 12 (54) |
| 28 days | |||
| Death | 2 (33) | 6 (38) | 14 (64) |
| Survivor | 4 (66) | 10 (62) | 8 (36) |
| 6 months | |||
| Death | 2 (33) | 6 (38) | 15 (68) |
| Survivor | 4 (66) | 10 (62) | 7 (32) |
| All Patients | No Sepsis | Sepsis | Septic Shock |
|---|---|---|---|
| n = 42 | n = 6 | n = 15 | n = 21 |
| LAG | 4.3 ± 1.5 | 4.5 ± 1.9 | 5.8 ± 2.0 |
| RAG | 4.7 ± 1.1 | 4.2 ± 1.7 | 4.5 ± 1.9 |
| Total | 9.0 ± 1.3 | 9.1 ± 1.9 | 10.8 ± 2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milberg, M.; Kindt, A.; Luft, L.; Hoffmann, U.; Behnes, M.; Schoenberg, S.O.; Janssen, S. Diagnostic and Prognostic Value of Quantitative Computed Tomography Parameters of Adrenal Glands in Patients from Internistled ICU with Sepsis and Septic Shock. Anatomia 2022, 1, 14-32. https://doi.org/10.3390/anatomia1010003
Milberg M, Kindt A, Luft L, Hoffmann U, Behnes M, Schoenberg SO, Janssen S. Diagnostic and Prognostic Value of Quantitative Computed Tomography Parameters of Adrenal Glands in Patients from Internistled ICU with Sepsis and Septic Shock. Anatomia. 2022; 1(1):14-32. https://doi.org/10.3390/anatomia1010003
Chicago/Turabian StyleMilberg, Moritz, Alida Kindt, Lisa Luft, Ursula Hoffmann, Michael Behnes, Stefan O. Schoenberg, and Sonja Janssen. 2022. "Diagnostic and Prognostic Value of Quantitative Computed Tomography Parameters of Adrenal Glands in Patients from Internistled ICU with Sepsis and Septic Shock" Anatomia 1, no. 1: 14-32. https://doi.org/10.3390/anatomia1010003