Early Detection of Phototrophic Biofilms in the Polychrome Panel, El Castillo Cave, Spain
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Site
2.2. Cave Monitoring
2.3. Sampling Site
2.4. Culture of Natural Samples
2.5. Extraction of Nucleic Acids
2.6. Sequencing
2.7. Bioinformatics
3. Results
3.1. Main Seasonal Pattern of Cave Environmental Conditions
3.2. Microbial Community Composition
3.3. Prokaryotes Community Structure
3.4. Eukaryotes Community Structure
4. Discussion
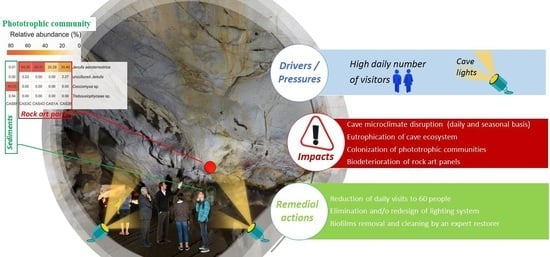
4.1. Microclimate Disruption by Daily Visitors and Cave Lighting
4.2. Prokaryota
4.3. Eukaryota
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saiz-Jimenez, C. The microbiology of show caves, mines tunnels and tombs: Implications for management and conservation. In Microbial Life of Cave Systems; Engel, A.S., Ed.; DeGruiter: Berlin, Germany, 2015; pp. 231–261. [Google Scholar]
- Jurado, V.; Novakova, A.; Hernandez-Marine, M.; Saiz-Jimenez, C. Cueva del Tesoro, Rincon de la Victoria, Malaga: A treasure of biodiversity. In The Conservation of Subterranean Cultural Heritage; Saiz-Jimenez, C., Ed.; CRC Press/Balkema: Leiden, The Netherlands, 2014; pp. 207–214. [Google Scholar]
- Asencio, A.D.; Aboal, M. A contribution to knowledge of chasmoendolithic algae in cave-like environments. Algol. Stud. 2000, 98, 133–151. [Google Scholar] [CrossRef]
- Czerwik-Marcinkowska, J.; Wojciechowska, A.; Massalski, A. Biodiversity of limestone caves: Aggregations of aerophytic algae and cyanobacteria in relation to site factors. Pol. J. Ecol. 2015, 63, 481–499. [Google Scholar] [CrossRef]
- Popović, S.; Nikolić, N.; Jovanović, J.; Predojević, D.; Trbojević, I.; Manić, L.J.; Simić, G.S. Cyanobacterial and algal abundance and biomass in cave biofilms and relation to environmental and biofilm parameters. Int. J. Speleol. 2019, 48, 49–61. [Google Scholar] [CrossRef]
- Smith, T.; Olson, R. A taxonomic survey of lamp flora (Algae and Cyanobacteria) in electrically lit passages within Mammoth Cave National Park, Kentucky. Int. J. Speleol. 2007, 36, 105–114. [Google Scholar] [CrossRef]
- Vinogradova, O.N.; Nevo, E.; Wasser, S.P. Algae of the Sefunim Cave (Israel): Species diversity affected by light, humidity and rock stresses. Int. J. Algae 2009, 11, 99–116. [Google Scholar] [CrossRef]
- Sánchez-Moral, S.; Soler, V.; Cañaveras, J.C.; Sanz, E.; Van Grieken, R.; Gysells, K. Inorganic deterioration affecting Altamira Cave. Quantitative approach to wall-corrosion (solution etching) processes induced by visitors. Sci. Total Environ. 1999, 243, 67–84. [Google Scholar] [CrossRef]
- Lamprinou, V.; Danielidis, D.B.; Economou-Amilli, A.; Pantazidou, A. Distribution survey of Cyanobacteria in three Greek caves of Peloponnese. Int. J. Speleol. 2012, 41, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Agnew, N.; Deacon, J.; Hall, N.; Little, T.; Sullivan, S.; Taçon, P. Rock Art. A Cultural Treasure at Risk; The Getty Conservation Institute: Los Angeles, CA, USA, 2015. [Google Scholar]
- Bastian, F.; Jurado, V.; Novakova, A.; Alabouvette, C.; Saiz-Jimenez, C. The microbiology of the Lascaux Cave. Microbiology 2010, 156, 644–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saiz-Jimenez, C.; Cuezva, S.; Jurado, V.; Fernandez-Cortes, A.; Porca, E.; Benavente, D.; Cañaveras, J.C.; Sanchez-Moral, S. Paleolithic art in peril: Policy and science collide at Altamira Cave. Science 2011, 334, 42–43. [Google Scholar] [CrossRef] [Green Version]
- Martin-Sanchez, P.; Miller, A.Z.; Saiz-Jimenez, C. Lascaux Cave: An example of fragile ecological balance in subterranean Environments. In Microbial Life of Cave Systems; Engel, A.S., Ed.; DeGruiter: Berlin, Germany, 2015; pp. 280–301. [Google Scholar]
- Cuezva, S.; Jurado, V.; Fernandez-Cortes, A.; Garcia-Anton, E.; Rogerio-Candelera, M.A.; Ariño, X.; Benavente, D.; Cañaveras, J.C.; Saiz-Jimenez, C.; Sanchez-Moral, S. Scientific data suggest Altamira Cave should remain closed. In Microbial Life of Cave Systems; Engel, A.S., Ed.; DeGruiter: Berlin, Germany, 2015; pp. 303–320. [Google Scholar]
- Lefèvre, M. La maladie verte de Lascaux. Stud. Conserv. 1974, 19, 126–156. [Google Scholar]
- Portillo, M.C.; Saiz-Jimenez, C.; Gonzalez, J.M. Molecular characterization of total and metabolically active bacterial communities of ‘‘white colonizations’’ in the Altamira Cave, Spain. Res. Microbiol. 2009, 160, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Alcalde del Río, H. Las Pinturas y Grabados de las Cavernas Prehistóricas de la Provincia de Santander (Altamira, Covalanas, Hornos de la Peña, Castillo); Blanchard y Arce: Santander, Spain, 1906. [Google Scholar]
- Alcalde del Río, H.; Breuil, H.; Sierra, L. Les Cavernes de la Région Cantabrique (Espagne); Imprimerie Vve. A. Chêne: Monaco, Monaco, 1911. [Google Scholar]
- Cabrera Valdés, V. El Yacimiento de la Cueva de "El Castillo" (Puente Viesgo, Santander); CSIC: Madrid, Spain, 1984.
- Breuil, H. Quatre Cents Siècles d’Art Pariétal. Les Cavernes Ornées de l’Age du Renne; Centre d’Études et de Documentation Préhistoriques: Montignac, France, 1952. [Google Scholar]
- Valladas, H.; Cachier, H.; Maurice, P.; Quirós, F.; Clottes, J.; Cabrera, M.V.; Uzquiano, P.; Arnold, M. Direct radiocarbon dates for prehistoric paintings at the Altamira, El Castillo and Niaux caves. Nature 1992, 357, 68–70. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.; Herdman, M.; Stanier, R. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 10 October 2021).
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2 paper supporting information: High-resolution sample inference from amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Opens external link in new window. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Caporaso, J.G. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Martin-Pozas, T.; Sanchez-Moral, S.; Cuezva, S.; Jurado, V.; Saiz-Jimenez, C.; Perez-Lopez, R.; Carrey, R.; Otero, N.; Giesemann, A.; Well, R.; et al. Biologically mediated release of endogenous N2O and NO2 gases in a hydrothermal, hypoxic subterranean environment. Sci. Total Environ. 2020, 747, 141218. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pimentel, J.L.; Martin-Pozas, T.; Jurado, V.; Miller, A.Z.; Caldeira, A.T.; Fernandez-Lorenzo, O.; Sanchez-Moral, S.; Saiz-Jimenez, C. Prokaryotic communities from a lava tube cave in La Palma Island (Spain) are involved in the biogeochemical cycle of major elements. PeerJ 2021, 9, e11386. [Google Scholar] [CrossRef] [PubMed]
- Itcus, C.; Pascu, M.D.; Lavin, P.; Persoiu, A.; Iancu, L.; Purcarea, C. Bacterial and archaeal community structures in perennial cave ice. Sci. Rep. 2018, 8, 15671. [Google Scholar] [CrossRef] [Green Version]
- Jurado, V.; del Rosal, Y.; Gonzalez-Pimentel, J.L.; Hermosin, B.; Saiz-Jimenez, C. Biological control of phototrophic biofilms in a show cave: The case of Nerja Cave. Appl. Sci. 2020, 10, 3448. [Google Scholar] [CrossRef]
- Jurado, V.; Gonzalez-Pimentel, J.L.; Miller, A.Z.; Hermosin, B.; D’Angeli, I.M.; Tognini, P.; De Waele, J.; Saiz-Jimenez, C. Microbial communities in vermiculation deposits from an Alpine cave. Front. Earth Sci. 2020, 8, 586248. [Google Scholar] [CrossRef]
- Addesso, R.; Gonzalez-Pimentel, J.; D’Angeli, I.M.; De Waele, J.; Saiz-Jimenez, C.; Jurado, V.; Miller, A.Z.; Cubero, B.; Vigliotta, G.; Baldantoni, D. Microbial community characterizing vermiculations from karst caves and its role in their formation. Microb. Ecol. 2021, 81, 884–896. [Google Scholar] [CrossRef]
- Bernardini, S.; Bellatreccia, F.; Columbu, A.; Vaccarelli, I.; Pellegrini, M.; Jurado, V.; Del Gallo, M.; Saiz-Jimenez, C.; Sodo, A.; Millo, C.; et al. Morpho- mineralogical and bio-geochemical description of cave manganese stromatolite-like patinas (Grotta del Cervo, Central Italy) and hints on their paleohydrological-driven genesis. Front. Earth Sci. 2021, 9, 642667. [Google Scholar] [CrossRef]
- Deeg, C.M.; Zimmer, M.M.; George, E.E.; Husnik, F.; Keeling, P.J.; Suttle, C.A. Chromulinavorax destructans, a pathogen of microzooplankton that provides a window into the enigmatic candidate phylum Dependentiae. PLoS Pathog. 2019, 15, e1007801. [Google Scholar] [CrossRef] [Green Version]
- Yun, Y.; Wang, H.; Man, B.; Xiang, X.; Zhou, J.; Qiu, X.; Duan, Y.; Engel, A.S. The relationship between pH and bacterial communities in a single karst ecosystem and its implication for soil acidification. Front. Microbiol. 2016, 7, 1955. [Google Scholar] [CrossRef]
- Yasir, M. Analysis of bacterial communities and characterization of antimicrobial strains from cave microbiota. Brasil. J. Microbiol. 2018, 49, 248–257. [Google Scholar] [CrossRef]
- Porca, E.; Jurado, V.; Žgur-Bertok, D.; Saiz-Jimenez, C.; Pašić, L. Comparative analysis of yellow microbial communities growing on the walls of geographically distinct caves indicates a common core of microorganisms involved in their formation. FEMS Microbiol. Ecol. 2012, 81, 255–266. [Google Scholar] [CrossRef]
- Gonzalez-Pimentel, J.L.; Miller, A.Z.; Jurado, V.; Laiz, L.; Pereira, M.F.C.; Saiz-Jimenez, C. Yellow colored mats from lava tube of La Palma (Canary Islands, Spain) are dominated by metabolically active Actinobacteria. Sci. Rep. 2018, 8, 1944. [Google Scholar] [CrossRef] [Green Version]
- Groth, I.; Vetermann, R.; Schuetze, B.; Schumann, P.; Saiz-Jimenez, C. Actinomycetes in kartic caves of Northern Spain (Altamira and Tito Bustillo). J. Microbiol. Methods 1999, 36, 115–122. [Google Scholar] [CrossRef]
- Cuezva, S.; Fernandez-Cortes, A.; Porca, E.; Pasic, L.; Jurado, V.; Hernandez-Marine, M.; Serrano-Ortiz, P.; Hermosin, B.; Cañaveras, J.C.; Sanchez-Moral, S.; et al. The biogeochemical role of Actinobacteria in Altamira Cave, Spain. FEMS Microbiol. Ecol. 2012, 81, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riquelme, C.; Hathaway, J.J.M.; Dapkevicius, M.L.N.E.; Miller, A.Z.; Kooser, A.; Northup, D.E.; Jurado, V.; Fernandez, O.; Saiz-Jimenez, C.; Cheeptham, N. Actinobacterial diversity in volcanic caves and associated geomicrobiological interactions. Front. Microbiol. 2015, 6, 1342. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Meade, A.; Lam, H.-M.; Luo, H. Evolutionary timeline and genomic plasticity underlying the lifestyle diversity in Rhizobiales. mSystems 2020, 5, e00438-20. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Herraiz, M.; Jurado, V.; Cuezva, S.; Laiz, L.; Pallecchi, P.; Tiano, P.; Sanchez-Moral, S.; Saiz-Jimenez, C. The actinobacterial colonization of Etruscan paintings. Sci. Rep. 2013, 3, 1440. [Google Scholar] [CrossRef] [Green Version]
- Gan, H.Y.; Gan, H.M.; Tarasco, A.M.; Busairi, N.I.; Barton, H.A.; Hudson, A.O.; Savka, M.A. Whole-genome sequences of five oligotrophic bacteria isolated from deep within Lechuguilla Cave, New Mexico. Genome Announc. 2014, 2, e01133-14. [Google Scholar] [CrossRef] [Green Version]
- Tisato, N.; Torriani, S.F.F.; Monteux, S.; Sauro, F.; De Waele, J.; Tavagna, M.L.; D’Angeli, I.M.; Chailloux, D.; Renda, M.; Eglinton, T.I.; et al. Microbial mediation of complex subterranean mineral structures. Sci. Rep. 2015, 5, 15525. [Google Scholar] [CrossRef] [Green Version]
- Alonso, L.; Creuzé-des-Châtelliers, C.; Trabac, T.; Dubost, A.; Moënne-Loccoz, Y.; Pommier, T. Rock substrate rather than black stain alterations drives microbial community structure in the passage of Lascaux Cave. Microbiome 2018, 6, 1–15. [Google Scholar] [CrossRef]
- Marques, E.L.S.; Silva, G.S.; Dias, J.C.T.; Gross, E.; Costa, M.S.; Rezende, R.R. Cave drip water-related samples as a natural environment for aromatic hydrocarbon degrading bacteria. Microorganisms 2019, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Mantelin, S.; Fischer-Le Saux, M.; Zakhia, F.; Béna, G.; Bonneau, S.; Jeder, H.; de Lajudie, P.; Cleyet-Marel, J.-C. Emended description of the genus Phyllobacterium and description of four novel species associated with plant roots: Phyllobacterium bourgognense sp. nov., Phyllobacterium ifriqiyense sp. nov., Phyllobacterium leguminum sp. nov. and Phyllobacterium brassicacearum sp. nov. Int. J. Syst. Evol. Microbiol. 2006, 56, 827–839. [Google Scholar]
- Jurado, V.; Laiz, L.; Gonzalez, J.M.; Hernandez-Marine, M.; Valens, M.; Saiz-Jimenez, C. Phyllobacterium catacumbae sp. nov., a member of the order ‘Rhizobiales’ isolated from Roman catacombs. Int. J. Syst. Evol. Microbiol. 2005, 55, 1487–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Sanchez, P.M.; Jurado, V.; Porca, E.; Bastian, F.; Lacanette, D.; Alabouvette, C.; Saiz-Jimenez, C. Airborne microorganisms in Lascaux Cave (France). Int. J. Speleol. 2014, 43, 295–303. [Google Scholar] [CrossRef]
- Dominguez-Moñino, I.; Jurado, V.; Rogerio-Candelera, M.A.; Hermosin, B.; Saiz-Jimenez, C. Airborne bacteria in show caves from Southern Spain. Microb. Cell 2021, 8, 247–255. [Google Scholar] [CrossRef]
- Diaz-Herraiz, M.; Jurado, V.; Cuezva, S.; Laiz, L.; Pallecchi, P.; Tiano, P.; Sanchez-Moral, S.; Saiz-Jimenez, C. Deterioration of an Etruscan tomb by bacteria from the order Rhizobiales. Sci. Rep. 2014, 4, 3610. [Google Scholar] [CrossRef] [Green Version]
- Reddy, G.S.N.; Garcia-Pichel, F. Dyadobacter crusticola sp. nov., from biological soil crusts in the Colorado Plateau, USA, and an emended description of the genus Dyadobacter Chelius and Triplett 2000. Int. J. Syst. Evol. Microbiol. 2005, 55, 1295–1299. [Google Scholar] [CrossRef] [Green Version]
- Suihko, M.-L.; Alakomi, H.-L.; Gorbushina, A.; Fortune, I.; Marquardt, J.; Saarela, M. Characterization of aerobic bacterial and fungal microbiota on surfaces of historic Scottish monuments. Syst. Appl. Microbiol. 2007, 30, 494–508. [Google Scholar] [CrossRef] [PubMed]
- Pagnier, I.; Yutin, N.; Croce, O.; Makarova, K.S.; Wolf, Y.I.; Benamar, S.; Raoult, D.; Koonin, E.V.; La Scola, B. Babela massiliensis, a representative of a widespread bacterial phylum with unusual adaptations to parasitism in amoebae. Biol. Direct 2015, 10, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayamajhee, B.; Subedi, D.; Peguda, H.K.; Willcox, M.D.; Henriquez, F.L.; Carnt, N.A. Systematic review of intracellular microorganisms within Acanthamoeba to understand potential impact for infection. Pathogens 2021, 10, 225. [Google Scholar] [CrossRef]
- Thompson, E.; Erickson, M.; Malik, N.; Mettler, R.; Reman, B.; Ren, Y.; Bergmann, D. Culture-independent characterization of “cave silver” biofilms from the 1470 m level of the Sanford Underground Research Facility, Lead, SD. Proc. South Dakota Acad. Sci. 2020, 99, 29–55. [Google Scholar]
- Bousbia, S.; Papazian, L.; Saux, P.; Forel, J.-M.; Auffray, J.-P.; Martin, C.; Raoult, D.; La Scola, B. Serologic prevalence of amoeba-associated microorganisms in intensive care unit pneumonia patients. PLoS ONE 2013, 8, e58111. [Google Scholar] [CrossRef] [Green Version]
- Horn, M.; Wagner, M.; Müller, K.-D.; Schmid, E.N.; Fritsche, T.R.; Schleifer, K.-H.; Michel, R. Neochlamydia hartmannellae gen. nov., sp. nov. (Parachlamydiaceae), an endoparasite of the amoeba Hartmannella vermiformis. Microbiology 2000, 146, 1231–1239. [Google Scholar] [CrossRef] [Green Version]
- Köstlbacher, S.; Michels, S.; Siegl, A.; Schulz, F.; Domman, D.; Jongwutiwes, S.; Putaporntip, C.; Horn, M.; Collingro, A. Draft genome sequences of Chlamydiales bacterium STE3 and Neochlamydia sp. strain AcF84, endosymbionts of Acanthamoeba spp. Microbiol. Resour. Announc. 2020, 9, e00220-20. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, M.; Całusinska, M.; Cornet, L.; Adam, D.; Pessi, I.S.; Malchair, S.; Delfosse, P.; Baurain, D.; Barton, H.A.; Carnol, M.; et al. High-throughput sequencing analysis of the actinobacterial spatial diversity in moonmilk deposits. Antibiotics 2018, 7, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagnier, I.; Raoult, D.; La Scola, B. Isolation and characterization of Reyranella massiliensis gen. nov., sp. nov. from freshwater samples by using an amoeba co-culture procedure. Int. J. Syst. Evol. Microbiol. 2011, 61, 2151–2154. [Google Scholar] [CrossRef] [Green Version]
- La Scola, B.; Mallet, M.-N.; Grimont, P.A.D.; Raoult, D. Bosea eneae sp. nov., Bosea massiliensis sp. nov. and Bosea vestrisii sp. nov., isolated from hospital water supplies, and emendation of the genus Bosea (Das et al. 1996). Int. J. Syst. Evol. Microbiol. 2003, 53, 15–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Scola, B.; Barrasi, L.; Raoult, D. A novel alpha-Proteobacterium, Nordella oligomobilis gen. nov., sp. nov., isolated by using amoebal co-cultures. Res. Microbiol. 2004, 155, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Bastian, F.; Alabouvette, C.; Saiz-Jimenez, C. Bacteria and free-living amoeba in the Lascaux Cave. Res. Microbiol. 2009, 160, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Thomas, V.; McDonell, G.; Denyer, S.P.; Maillard, J.-Y. Free-living amoebae and their intracellular pathogenic microorganisms: Risks for water quality. FEMS Microbiol. Rev. 2010, 34, 231–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Sanchez, A.M.; Ariza, C.; Ubeda, J.M.; Martin-Sanchez, P.M.; Jurado, V.; Bastian, F.; Alabouvette, C.; Saiz-Jimenez, C. Free-living amoebae in sediments from the Lascaux Cave in France. Int. J. Speleol. 2013, 42, 9–13. [Google Scholar] [CrossRef]
- Taylor-Mulneix, D.L.; Soumana, I.H.; Linz, B.; Harvill, E.T. Evolution of Bordetellae from environmental microbes to human respiratory pathogens: Amoebae as a missing link. Front. Cell. Infect. Microbiol. 2017, 7, 510. [Google Scholar] [CrossRef] [PubMed]
- Groth, I.; Schumann, P.; Laiz, L.; Sanchez-Moral, S.; Cañaveras, J.C.; Saiz-Jimenez, C. Geomicrobiological study of the Grotta dei Cervi, Porto Badisco, Italy. Geomicrobiol. J. 2001, 18, 241–258. [Google Scholar] [CrossRef]
- Schabereiter-Gurtner, C.; Saiz-Jimenez, C.; Piñar, G.; Lubitz, W.; Rolleke, S. Phylogenetic diversity of bacteria associated with Paleolithic paintings and surrounding rock walls in two Spanish caves (Llonin and La Garma). FEMS Microbiol. Ecol. 2004, 47, 235–247. [Google Scholar] [CrossRef]
- Van der Kooij, D.; Veenendaal, H.R.; Italiaander, R.; van der Mark, E.J.; Dignum, M. Primary colonizing Betaproteobacteriales play a key role in the growth of Legionella pneumophila in biofilms on surfaces exposed to drinking water treated by slow sand filtration. Appl. Environ. Microbiol. 2018, 84, e01732-18. [Google Scholar] [CrossRef] [Green Version]
- Cañaveras, J.C.; Sanchez-Moral, S.; Soler, V.; Saiz-Jimenez, C. Microorganisms and microbially induced fabrics in cave walls. Geomicrobiol. J. 2001, 18, 223–240. [Google Scholar]
- Jurado, V.; Laiz, L.; Sanchez-Moral, S.; Saiz-Jimenez, C. Pathogenic microorganisms related to human visits in Altamira Cave, Spain. In The Conservation of Subterranean Cultural Heritage; Saiz-Jimenez, C., Ed.; CRC Press/Balkema: Leiden, The Netherlands, 2014; pp. 229–238. [Google Scholar]
- Aguilar, M.; Lado, C.; Spiegel, F.W. Protostelids from deciduous forests: First data from southwestern Europe. Mycol. Res. 2007, 111, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.; Spiegel, E.W.; Lado, C. Microhabitat and climatic preferences of protosteloid amoebae in a region with a Mediterranean climate. Microb. Ecol. 2011, 62, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Procházková, K.; Němcová, Y.; Neustupa, J. A new species Jenufa aeroterrestrica (Chlorophyceae incertae sedis, Viridiplantae), described from Europe. Preslia 2015, 87, 403–416. [Google Scholar]
- Soares, F.; Portugal, A.; Trovão, J.; Coelho, C.; Mesquita, N.; Pinheiro, A.C.; Gil, F.; Catarino, L.; Cardoso, S.M.; Tiago, I. Structural diversity of photoautotrophic populations within the UNESCO site “Old Cathedral of Coimbra” (Portugal), using a combined approach. Int. Biodeter. Biodegr. 2019, 140, 9–20. [Google Scholar] [CrossRef]
- Hallmann, C.; Stannek, L.; Fritzlar, D.; Hause-Reitner, D.; Friedl, T.; Hoppert, M. Molecular diversity of phototrophic biofilms on building stone. FEMS Microbiol. Ecol. 2013, 84, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Hodač, L.; Hallmann, C.; Rosenkranz, H.; Faßhauer, F.; Friedl, T. Molecular evidence for the wide distribution of two lineages of terrestrial green algae (Chlorophyta) over tropics to temperate zone. ISRN Ecol. 2012, 12, 795924. [Google Scholar] [CrossRef] [Green Version]
- Del Rosal Padial, Y.; Jurado Lobo, V.; Hernández-Mariné, M.; Roldán Molina, M.; Sáiz-Jiménez, C. Biofilms en cuevas turísticas: La Cueva de Nerja y la Cueva del Tesoro. In El Karst y el Hombre: Las Cuevas como Patrimonio Mundial; Andreo, B., Durán, J.J., Eds.; Asociación de Cuevas Turísticas Españolas: Madrid, Spain, 2016; pp. 103–114. [Google Scholar]
- Song, H.; Li, S.; Liu, X.; Wang, Q.; Zhu, H.; Liu, G.; Hu, Z. Jenufa lobulosa sp. nov. (Chlorophyceae, Chlorophyta), a new epilithic, terrestrial species described from China. Phycologia 2017, 57, 52–60. [Google Scholar] [CrossRef]
- Kol, E. Algal growth experiments in the Baradla cave at Aggletek. Int. J. Speleol. 1967, 2, 457–474. [Google Scholar] [CrossRef] [Green Version]
- Zammit, G. Phototrophic biofilm communities and adaptation to growth on ancient archaeological surfaces. Ann. Microbiol. 2019, 69, 1047–1058. [Google Scholar] [CrossRef]
- Darienko, T.; Gustavs, L.; Eggert, A.; Wolf, W.; Pröschold, T. Evaluating the species boundaries of green microalgae (Coccomyxa, Trebouxiophyceae, Chlorophyta) using integrative taxonomy and DNA barcoding with further implications for the species identification in environmental samples. PLoS ONE 2015, 10, e0127838. [Google Scholar] [CrossRef] [PubMed]
- Zoller, S.; Lutzoni, F. Slow algae, fast fungi: Exceptionally high nucleotide substitution rate differences between lichenized fungi Omphalina and their symbiotic green algae Coccomyxa. Mol. Phylogenet. Evol. 2003, 29, 629–640. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Falagán, C.; Ayala, D.; Wendt-Potthoff, K. Adaptation of Coccomyxa sp. to extremely low light conditions causes deep chlorophyll and oxygen maxima in acidic pit lakes. Microorganisms 2020, 8, 1218. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.J. Algological investigations in Mammoth Cave, Kentucky. Int. J. Speleol. 1965, 1, 491–516. [Google Scholar] [CrossRef] [Green Version]
- Popkova, A.; Mazina, S.; Lashenova, T. Phototrophic communities of Ahshtyrskaya Cave in the condition of artificial light. Ecol. Monteneg. 2019, 23, 8–19. [Google Scholar] [CrossRef] [Green Version]
- Czerwik-Marcinkowska, J.; Galczynska, K.; Oszczudlowski, J.; Massalski, A.; Semaniak, J.; Arabski, M. Fatty acid methyl esters of the aerophytic cave alga Coccomyxa subglobosa as a source for biodiesel production. Energies 2020, 13, 6494. [Google Scholar] [CrossRef]
- Mulec, J.; Kosi, G.; Vrhovšek, D. Characterization of cave aerophytic algal communities and effects of irradiance levels on production of pigments. J. Caves Karst Stud. 2008, 70, 3–12. [Google Scholar]
- Piano, E.; Bona, F.; Falasco, E.; La Morgia, V.; Badino, G.; Isaia, M. Environmental drivers of phototrophic biofilms in an Alpine show cave (SW-Italian Alps). Sci. Total Environ. 2015, 536, 1007–1018. [Google Scholar] [CrossRef]
- Muñoz-Fernández, J.; Del Rosal, Y.; Álvarez-Gómez, F.; Hernández-Mariné, M.; Guzmán-Sepúlveda, R.; Korbee, N.; Figueroa, F.L. Selection of LED lighting systems for the reduction of the biodeterioration of speleothems induced by photosynthetic biofilms in the Nerja Cave (Malaga, Spain). J. Photochem. Photobiol. B Biol. 2021, 217, 112155. [Google Scholar] [CrossRef]
- Abe, K.; Ishiwatari, T.; Wakamatsu, M.; Aburai, N. Fatty acid content and profile of the aerial microalga Coccomyxa sp. isolated from dry environments. Appl. Biochem. Biotechnol. 2014, 174, 1724–1735. [Google Scholar] [CrossRef] [PubMed]
- Karunarathna, S.C.; Dong, Y.; Karasaki, S.; Tibpromma, S.; Hyde, K.D.; Lumyong, S.; Xu, J.; Sheng, J.; Mortimer, P.E. Discovery of novel fungal species and pathogens on bat carcasses in a cave in Yunnan Province, China. Emerg. Microb. Infect. 2020, 9, 1554–1566. [Google Scholar] [CrossRef] [PubMed]
- Jurado, V.; Porca, E.; Cuezva, S.; Fernandez-Cortes, A.; Sanchez-Moral, S.; Saiz-Jimenez, C. Fungal outbreak in a show cave. Sci. Total Environ. 2010, 408, 3632–3638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delafont, V.; Rodier, M.-H.; Maisonneuve, E.; Cateau, E. Vermamoeba vermiformis: A free-living amoeba of interest. Microb. Ecol. 2018, 76, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Walochnik, J.; Mulec, J. Free-living amoebae in carbonate precipitating microhabitats of karst caves and a new vahlkampfiid amoeba, Allovahlkampfia spelaea gen. nov., sp. nov. Acta Protozool. 2009, 48, 25–33. [Google Scholar]
- Mazei, Y.; Belyakova, O.; Trulova, A.; Guidolin, L.; Coppellotti, O. Testate amoebae communities from caves of some territories in European Russia and North-Eastern Italy. Protistology 2012, 7, 42–50. [Google Scholar]
- Pickup, Z.L.; Pickup, R.; Parry, J.D. Effects of bacterial prey species and their concentration on growth of the amoebae Acanthamoeba castellanii and Hartmannella vermiformis. Appl. Environ. Microbiol. 2007, 73, 2631–2634. [Google Scholar] [CrossRef] [Green Version]
- Smirnov, A.V.; Chao, E.; Nassonova, E.S.; Cavalier-Smith, T. A revised classification of naked lobose amoebae (Amoebozoa: Lobosa). Protist 2011, 162, 545–570. [Google Scholar] [CrossRef]
- Kudryavtsev, A.; Pawlowski, J. Cunea n. g. (Amoebozoa, Dactylopodida) with two cryptic species isolated from different areas of the ocean. Eur. J. Protistol. 2015, 51, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Zare, R.; Gams, W.; Starink-Willemse, M.; Summerbell, R.C. Gibellulopsis, a suitable genus for Verticillium nigrescens, and Musicillium, a new genus for V. theobromae. Nova Hedwigia 2007, 85, 463–489. [Google Scholar] [CrossRef] [Green Version]
- Pitt, J.I. Geosmithia gen. nov. for Penicillium lavendulum and related species. Can. J. Bot. 1979, 57, 2021–2030. [Google Scholar] [CrossRef]
- Kolařík, M.; Kubátová, A.; Pažoutová, S.; Šrûtka, P. Morphological and molecular characterisation of Geosmithia putterillii, G. pallida comb. nov. and G. flava sp. nov., associated with subcorticolous insects. Mycol. Res. 2004, 108, 1053–1069. [Google Scholar] [CrossRef] [PubMed]
- Bastian, F.; Alabouvette, C.; Saiz-Jimenez, C. The impact of arthropods on fungal community structure in Lascaux Cave. J. Appl. Microbiol. 2009, 106, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Esteban Pérez, R. Study and remediation of environmental problems caused due to the growth of algae in speleothems of calcareous caves adapted for tourism- a case of success in Spain. J. Environ. Geol. 2018, 2, 20–27. [Google Scholar]
- Sanmartín, P. New perspectives against biodeterioration through public lighting. In Microorganisms in the Deterioration and Preservation of Cultural Heritage; Joseph, E., Ed.; Springer: Cham, Switzerland, 2021; pp. 155–171. [Google Scholar]
- Mulec, J.; Kosi, G. Lampenflora algae and methods of growth control. J. Cave Karst Stud. 2009, 71, 109–115. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurado, V.; Gonzalez-Pimentel, J.L.; Fernandez-Cortes, A.; Martin-Pozas, T.; Ontañon, R.; Palacio, E.; Hermosin, B.; Sanchez-Moral, S.; Saiz-Jimenez, C. Early Detection of Phototrophic Biofilms in the Polychrome Panel, El Castillo Cave, Spain. Appl. Biosci. 2022, 1, 40-63. https://doi.org/10.3390/applbiosci1010003
Jurado V, Gonzalez-Pimentel JL, Fernandez-Cortes A, Martin-Pozas T, Ontañon R, Palacio E, Hermosin B, Sanchez-Moral S, Saiz-Jimenez C. Early Detection of Phototrophic Biofilms in the Polychrome Panel, El Castillo Cave, Spain. Applied Biosciences. 2022; 1(1):40-63. https://doi.org/10.3390/applbiosci1010003
Chicago/Turabian StyleJurado, Valme, Jose Luis Gonzalez-Pimentel, Angel Fernandez-Cortes, Tamara Martin-Pozas, Roberto Ontañon, Eduardo Palacio, Bernardo Hermosin, Sergio Sanchez-Moral, and Cesareo Saiz-Jimenez. 2022. "Early Detection of Phototrophic Biofilms in the Polychrome Panel, El Castillo Cave, Spain" Applied Biosciences 1, no. 1: 40-63. https://doi.org/10.3390/applbiosci1010003