Potassium Nitrate Treatment Is Associated with Modulation of Seed Water Uptake, Antioxidative Metabolism and Phytohormone Levels of Pea Seedlings
Abstract
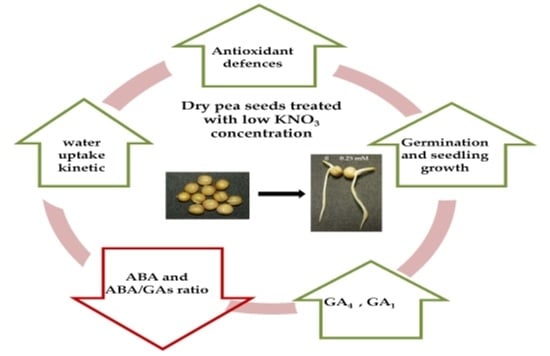
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Culture Conditions, Growth Measurements and Sampling
2.2. Enzyme Extraction and Assays
2.3. Ascorbate and Glutathione Analyses
2.4. Analysis of Plant Hormones
2.5. Statistical Analyses
3. Results
4. Discussion
4.1. Antioxidant Metabolism
4.2. Plant Hormones
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef] [Green Version]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Matsushima, K.-I.; Sakagami, J.-I. Effects of Seed Hydropriming on Germination and Seedling Vigor during Emergence of Rice under Different Soil Moisture Conditions. Am. J. Plant Sci. 2013, 4, 1584–1593. [Google Scholar] [CrossRef] [Green Version]
- Forti, C.; Ottobrino, V.; Bassolino, L.; Toppino, L.; Rotino, G.L.; Pagano, A.; Macovei, A.; Balestrazzi, A. Molecular dynamics of pre-germinative metabolism in primed eggplant (Solanum melongena L.) seeds. Hortic. Res. 2020, 7, 87. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, W.; He, J.; Zhang, L.; Wei, Y.; Yang, M. Nitric oxide alleviates salt stress in seed germination and early seedling growth of pakchoi (Brassica chinensis L.) by enhancing physiological and biochemical parameters. Ecotoxicol. Environ. Saf. 2020, 187, 109785. [Google Scholar] [CrossRef] [PubMed]
- Barba-Espin, G.; Diaz-Vivancos, P.; Clemente-Moreno, M.J.; Albacete, A.; Faize, L.; Faize, M.; Pérez-Alfocea, F.; Hernández, J.A. Interaction between hydrogen peroxide and plant hormones during germination and the early growth of pea seedlings. Plant Cell Environ. 2010, 33, 981–994. [Google Scholar] [CrossRef]
- Barba-Espín, G.; Diaz-Vivancos, P.; Job, D.; Belghazi, M.; Job, C.; Hernández, J.A. Understanding the role of H2O2 during pea seed germination: A combined proteomic and hormone profiling approach. Plant Cell Environ. 2011, 34, 1907–1919. [Google Scholar] [CrossRef]
- Barba-Espin, G.; Nicolas, E.; Almansa, M.S.; Cantero-Navarro, E.; Albacete, A.; Hernandez, J.A.; Diaz-Vivancos, P. Role of thioproline on seed germination: Interaction ROS-ABA and effects on antioxidative metabolism. Plant Physiol. Biochem. 2012, 59, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Bethke, P.C.; Libourel, I.G.L.; Jones, R.L. Nitric oxide reduces seed dormancy in Arabidopsis. J. Exp. Bot. 2006, 57, 517–526. [Google Scholar] [CrossRef]
- Anosheh, H.P.; Sadeghi, H.; Emam, Y. Chemical priming with urea and KNO3 enhances maize hybrids (Zea mays L.) seed viability under abiotic stress. J. Crop Sci. Biotechnol. 2011, 14, 289–295. [Google Scholar] [CrossRef]
- Lara, T.S.; Lira, J.M.S.; Rodrigues, A.C.; Rakocevic, M.; Alvarenga, A.A. Potassium Nitrate Priming Affects the Activity of Nitrate Reductase and Antioxidant Enzymes in Tomato Germination. J. Agric. Sci. 2014, 6, 72. [Google Scholar] [CrossRef]
- Vidal, A.; Cantabella, D.; Bernal-Vicente, A.; Díaz-Vivancos, P.; Hernández, J.A. Nitrate- and nitric oxide-induced plant growth in pea seedlings is linked to antioxidative metabolism and the ABA/GA balance. J. Plant Physiol. 2018, 230, 13–20. [Google Scholar] [CrossRef]
- Shim, S.I.; Moon, J.-C.; Jang, C.S.; Raymer, P.; Kim, W. Effect of Potassium Nitrate Priming on Seed Germination of Seashore Paspalum. HortScience 2008, 43, 2259–2262. [Google Scholar] [CrossRef] [Green Version]
- Duermeyer, L.; Khodapanahi, E.; Yan, D.; Krapp, A.; Rothstein, S.J.; Nambara, E. Regulation of seed dormancy and germination by nitrate. Seed Sci. Res. 2018, 28, 150–157. [Google Scholar] [CrossRef]
- Matakiadis, T.; Alboresi, A.; Jikumaru, Y.; Tatematsu, K.; Pichon, O.; Renou, J.P.; Kamiya, Y.; Nambara, E.; Truong, H.N. The Arabidopsis abscisic acid catabolic gene CYP707A2 plays a key role in nitrate control of seed dormancy. Plant Physiol. 2009, 149, 949–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chahtane, H.; Kim, W.; Lopez-Molina, L. Primary seed dormancy: A temporally multilayered riddle waiting to be unlocked. J. Exp. Bot. 2017, 68, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Iwabuchi, M. A mechanism for promoting the germination of Zinnia elegans seeds by hydrogen peroxide. Plant Cell Physiol. 2001, 42, 286–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ye, N.; Liu, R.; Chen, M.; Zhang, J. H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J. Exp. Bot. 2010, 61, 2979–2990. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, M.; Kuwahara, A.; Seo, M.; Kushiro, T.; Asami, T.; Hirai, N.; Kamiya, Y.; Koshiba, T.; Nambara, E. CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol. 2006, 141, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, M.; Hanada, A.; Yamauchi, Y.; Kuwahara, A.; Kamiya, Y.; Yamaguchi, S. Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 2003, 15, 1591–1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonogaki, H. Seed biology updates—Highlights and new discoveries in seed dormancy and germination research. Front. Plant Sci. 2017, 8, 524. [Google Scholar] [CrossRef] [Green Version]
- Beligni, M.V.; Lamattina, L. Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 2000, 210, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Rosental, L.; Nonogaki, H.; Fait, A. Activation and regulation of primary metabolism during seed germination. Seed Sci. Res. 2014, 24, 1–15. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foyer, C.H.; Ruban, A.V.; Noctor, G. Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem. J. 2017, 474, 877–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Vivancos, P.; Barba-Espín, G.; Hernández, J.A. Elucidating hormonal/ROS networks during seed germination: Insights and perspectives. Plant Cell Rep. 2013, 32, 1491–1502. [Google Scholar] [CrossRef]
- Gomes, M.P.; Garcia, Q.S. Reactive oxygen species and seed germination. Biologia 2013, 68, 351–357. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Kasa, S.; Sakamoto, M.; Aoki, N.; Kai, K.; Yuasa, T.; Hanada, A.; Yamaguchi, S.; Iwaya-Inoue, M. A Role for Reactive Oxygen Species Produced by NADPH Oxidases in the Embryo and Aleurone Cells in Barley Seed Germination. PLoS ONE 2015, 10, e0143173. [Google Scholar] [CrossRef]
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Different modes of hydrogen peroxide action during seed germination. Front. Plant Sci. 2016, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Bailly, C. The signalling role of ROS in the regulation of seed germination and dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef]
- Diaz-Vivancos, P.; Faize, M.; Barba-Espin, G.; Faize, L.; Petri, C.; Hernández, J.A.; Burgos, L. Ectopic expression of cytosolic superoxide dismutase and ascorbate peroxidase leads to salt stress tolerance in transgenic plums. Plant Biotechnol. J. 2013, 11, 976–985. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bueso, E.; Serrano, R.; Pallás, V.; Sánchez-Navarro, J.A. Seed tolerance to deterioration in arabidopsis is affected by virus infection. Plant Physiol. Biochem. 2017, 116, 1–8. [Google Scholar] [CrossRef]
- Hilhorst, H.W.M.; Smitt, A.I.; Karssen, C.M. Gibberellin-biosynthesis and -sensitivity mediated stimulation of seed germination of Sisymbrium officinale by red light and nitrate. Physiol. Plant. 1986, 67, 285–290. [Google Scholar] [CrossRef]
- Footitt, S.; Huang, Z.; Clay, H.A.; Mead, A.; Finch-Savage, W.E. Temperature, light and nitrate sensing coordinate Arabidopsis seed dormancy cycling, resulting in winter and summer annual phenotypes. Plant J. 2013, 74, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Manz, B.; Müller, K.; Kucera, B.; Volke, F.; Leubner-Metzger, G. Water uptake and distribution in germinating tobacco seeds investigated in vivo by nuclear magnetic resonance imaging. Plant Physiol. 2005, 138, 1538–1551. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Motos, J.R.; Noguera-Vera, L.; Barba-Espín, G.; Piqueras, A.; Hernández, J.A. Antioxidant metabolism and chlorophyll fluorescence during the acclimatisation to Ex vitro conditions of micropropagated Stevia rebaudiana bertoni plants. Antioxidants 2019, 8, 615. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Vivancos, P.; Clemente-Moreno, M.J.; Rubio, M.; Olmos, E.; García, J.A.; Martínez-Gómez, P.; Hernández, J.A. Alteration in the chloroplastic metabolism leads to ROS accumulation in pea plants in response to plum pox virus. J. Exp. Bot. 2008, 59, 2147–2160. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Vivancos, P.; Barba-Espín, G.; Clemente-Moreno, M.J.; Hernández, J.A. Characterization of the antioxidant system during the vegetative development of pea plants. Biol. Plant. 2010, 54, 76–82. [Google Scholar] [CrossRef]
- Kvaratskhelia, M.; Winkel, C.; Naldrett, M.T.; Thorneley, R.N.F. A novel high activity cationic ascorbate peroxidase from tea (Camellia sinensis)—A class III peroxidase with unusual substrate specificity. J. Plant Physiol. 1999, 154, 273–282. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Kamiya, Y.; Sun, T.P. Distinct cell-specific expression patterns of early and late gibberellin biosynthetic genes during Arabidopsis seed germination. Plant J. 2001, 28, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Barba-Espín, G.; Hernández, J.A.; Diaz-Vivancos, P. Role of H2O2 in pea seed germination. Plant Signal Behav. 2012, 7, 193–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. Comptes Rendus Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef]





| KNO3 (mM) | AOX | APX | MDHAR | DHAR | GR | POX | SOD |
|---|---|---|---|---|---|---|---|
| 0 | 60.5 ± 4.1 | 39.9 ± 4.2 a | 198 ± 12.9 b | 0.52 ± 0.05 b | 50.8 ± 3.0 b | 1383 ± 172 ab | 34.8 ± 2.5 |
| 0.25 | 51.9 ± 1.7 | 34.8 ± 2.2 a | 316 ± 15.9 a | 0.80 ± 0.08 a | 85.8 ± 3.1 a | 1765 ± 243 a | 37.5 ± 3.8 |
| 40 | 53.1 ± 3.8 | 18.2 ± 2.2 b | 165 ± 19.9 b | 0.85 ± 0.04 a | 80.6 ± 3.9 a | 713 ± 43 b | 27.7 ± 2.8 |
| a F | 2.39 ns | 13.7 ** | 23.4 *** | 23.4 *** | 31.7 *** | 8.46 ** | 2.71 ns |
| KNO3 (mM) | ASC | DHA | GSH | GSSG | GSH/ GSH + GSSG | Total Glutathione |
|---|---|---|---|---|---|---|
| 0 | 1401 ± 73 a | nd | 390 ± 19 a | 20.16 ± 3.12 a | 0.951 | 425 ± 32 a |
| 0.25 | 535 ± 34 b | nd | 263 ± 14 b | 9.27 ± 2.06 c | 0.966 | 281 ± 11 b |
| 40 | 375 ± 85 b | 7.60 ± 4.39 | 447 ± 26 a | 14.10 ± 1.81 b | 0.969 | 452 ± 29 a |
| a F | 60.9 *** | 32.9 *** | 21.7 *** | 15.2 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, J.A.; Díaz-Vivancos, P.; Acosta-Motos, J.R.; Barba-Espín, G. Potassium Nitrate Treatment Is Associated with Modulation of Seed Water Uptake, Antioxidative Metabolism and Phytohormone Levels of Pea Seedlings. Seeds 2022, 1, 5-15. https://doi.org/10.3390/seeds1010002
Hernández JA, Díaz-Vivancos P, Acosta-Motos JR, Barba-Espín G. Potassium Nitrate Treatment Is Associated with Modulation of Seed Water Uptake, Antioxidative Metabolism and Phytohormone Levels of Pea Seedlings. Seeds. 2022; 1(1):5-15. https://doi.org/10.3390/seeds1010002
Chicago/Turabian StyleHernández, José A., Pedro Díaz-Vivancos, José Ramón Acosta-Motos, and Gregorio Barba-Espín. 2022. "Potassium Nitrate Treatment Is Associated with Modulation of Seed Water Uptake, Antioxidative Metabolism and Phytohormone Levels of Pea Seedlings" Seeds 1, no. 1: 5-15. https://doi.org/10.3390/seeds1010002