The Energetics and Topology of Grain Boundaries in Magnesium: An Ab Initio Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grain Boundary Crystallography
2.2. Simulation Cell Construction
2.3. Calculation Details
2.4. Addition of Solutes
- (1)
- One solute atom at five different locations along the boundary
- (2)
- Five solute atoms all located at the boundary
- (3)
- Grain boundary behaviour at a concentration of 5% (+/0.3%)
- (1)
- 1 Zn and 1 Gd atom at the boundary
- (2)
- 2 Zn and 2 Gd atoms located at the boundary
3. Results
3.1. Pur Magnesium
3.2. Effect of Solutes
4. Discussion
4.1. Effct of Topology
4.2. Effect of Solute on GB Energetics
5. Conclusions
- The grain boundaries were found to have an intrinsic grain boundary energy, and this energy was not markedly affected by the solute concentration or chemistry at the boundary.
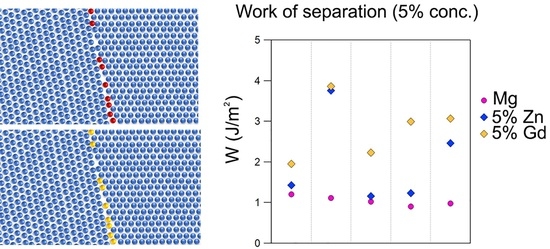
- In contrast with grain boundary energy, the work of separation (WSEP) was very sensitive to grain boundary chemistry. This parameter was therefore used to interrogate the effect of solute on grain boundary behaviour. It was found that for a boundary solute concentration of 5 at%, the effect of Zn and Gd on the WSEP was markedly different for the different boundaries. This is indicative that the two solutes will have a different effect on different boundary types.
- The topology of the grain boundary was correlated with the boundary energetics using the coordination number. For pure magnesium, the work of separation was found to correlate with coordination number, with more disordered boundaries of low CN showing higher values of WSEP. The effect of solute was not directly correlated with the CN, but there was a general trend for Gd to increase the WSEP more in those boundaries of low CN (high disorder).
- The WSEP was found to increase with increasing boundary solute concentration, with the rate of change being markedly different between the different boundaries. The increase in WSEP with solute concentration was typically higher for Gd compared to Zn.
- No correlation between the boundary behaviour and crystallography could be found, apart from the over-riding conclusion that all six boundaries showed different behaviours, and the effect of solute on their WSEP were unique.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacPherson, R.D.; Srolovitz, D.J. The von Neumann relation generalized to coarsening of three-dimensional microstructures. Nature 2007, 446, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Glicksman, M.E. Analysis of 3-D network structures. Philos. Mag. 2005, 85, 3–31. [Google Scholar] [CrossRef]
- Mahjoub, R.; Laws, K.J.; Stanford, N.; Ferry, M. General trends between solute segregation tendency and grain boundary character in aluminum—An ab inito study. Acta Mater. 2018, 158, 257–268. [Google Scholar] [CrossRef]
- Sutton, A.P.; Balluffi, R.W. Interfaces in Crystalline Materials; Oxford University Press: New York, NY, USA, 1996. [Google Scholar]
- Lejcek, P. Grain Boundary Segregaion in Metals; Springer: Berlin, Germany, 2010. [Google Scholar]
- Rohrer, G.S. Grain boundary energy anisotropy: A review. J. Mater. Sci. 2011, 46, 5881–5895. [Google Scholar] [CrossRef] [Green Version]
- Stanford, N.; Sabirov, I.; Sha, G.; la Fontaine, A.; Ringer, S.P.; Barnett, M.R. Effect of Al and Gd Solutes on the Strain Rate Sensitivity of Magnesium Alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2010, 41A, 734–743. [Google Scholar] [CrossRef]
- Nie, J.F.; Zhu, Y.M.; Liu, J.Z.; Fang, X.Y. Periodic Segregation of Solute Atoms in Fully Coherent Twin Boundaries. Science 2013, 340, 957–960. [Google Scholar] [CrossRef]
- Bugnet, M.; Kula, A.; Niewczas, M.; Botton, G.A. Segregation and clustering of solutes at grain boundaries in Mg-rare earth solid solutions. Acta Mater. 2014, 79, 66–73. [Google Scholar] [CrossRef]
- Hadorn, J.P.; Sasaki, T.T.; Nakata, T.; Ohkubo, T.; Kamado, S.; Hono, K. Solute clustering and grain boundary segregation in extruded dilute Mg-Gd alloys. Scr. Mater. 2014, 93, 28–31. [Google Scholar] [CrossRef] [Green Version]
- Robson, J.D.; Haigh, S.J.; Davis, B.; Griffiths, D. Grain Boundary Segregation of Rare-Earth Elements in Magnesium Alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2016, 47A, 522–530. [Google Scholar] [CrossRef] [Green Version]
- Basu, I.; Pradeep, K.G.; Miessen, C.; Barrales-Mora, L.A.; Al-Samman, T. The role of atomic scale segregation in designing highly ductile magnesium alloys. Acta Mater. 2016, 116, 77–94. [Google Scholar] [CrossRef]
- Stanford, N.; Sha, G.; Xia, J.H.; Ringer, S.P.; Barnett, M.R. Solute segregation and texture modification in an extruded magnesium alloy containing gadolinium. Scr. Mater. 2011, 65, 919–921. [Google Scholar] [CrossRef]
- Barrett, C.D.; Imandoust, A.; el Kadiri, H. The effect of rare earth element segregation on grain boundary energy and mobility in magnesium and ensuing texture weakening. Scr. Mater. 2018, 146, 46–50. [Google Scholar] [CrossRef]
- Rossouw, D.; Langelier, B.; Scullion, A.; Danaie, M.; Botton, G.A. Multivariate-aided mapping of rare-earth partitioning in a wrought magnesium alloy. Scr. Mater. 2016, 124, 174–178. [Google Scholar] [CrossRef]
- Trang, T.T.T.; Zhang, J.H.; Kim, J.H.; Zargaran, A.; Hwang, J.H.; Suh, B.C.; Kim, N.J. Designing a magnesium alloy with high strength and high formability. Nat. Commun. 2018, 9, 2522. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.M.; Bian, M.Z.; Nie, J.F. Tilt boundaries and associated solute segregation in a Mg-Gd alloy. Acta Mater. 2017, 127, 505–518. [Google Scholar] [CrossRef]
- Stanford, N.; Atwell, D.; Barnett, M.R. The effect of Gd on the recrystallisation, texture and deformation behaviour of magnesium-based alloys. Acta Mater. 2010, 58, 6773–6783. [Google Scholar] [CrossRef]
- Mahjoub, R.; Stanford, N. The electronic origins of the “rare earth” texture effect in magnesium alloys. Sci. Rep. 2021, 11, 14159. [Google Scholar] [CrossRef]
- Li, L.; Kamachali, R.D.; Li, Z.; Zhang, Z. Grain boundary energy effect on grain boundary segregation in an equiatomic high-entropy alloy. Phys. Rev. Mater. 2020, 4, 053603. [Google Scholar] [CrossRef]
- Lazar, E.A.; Mason, J.K.; MacPherson, R.D.; Srolovitz, D.J. Complete Topology of Cells, Grains, and Bubbles in Three-Dimensional Microstructures. Phys. Rev. Lett. 2012, 109, 095505. [Google Scholar] [CrossRef] [Green Version]
- Saylor, D.M.; Morawiec, A.; Rohrer, G.S. Distribution of grain boundaries in magnesia as a function of five macroscopic parameters. Acta Mater. 2003, 51, 3663–3674. [Google Scholar] [CrossRef]
- Tsuru, T.; Somekawa, H.; Chrzan, D.C. Interfacial segregation and fracture in Mg-based binary alloys: Experimental and first-principles perspective. Acta Mater. 2018, 151, 78–86. [Google Scholar] [CrossRef]
- Scheiber, D.; Pippan, R.; Puschnig, P.; Romaner, L. Ab initio calculations of grain boundaries in bcc metals. Model. Simul. Mater. Sci. Eng. 2016, 24, 035013. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Mahjoub, R.; Ferry, M.; Stanford, N. Local topology and its effects on grain boundary and solute segregation in HCP magnesium. Materialia 2019, 6, 100258. [Google Scholar] [CrossRef]
- Hase, T.; Ohtagaki, T.; Yamaguchi, M.; Ikeo, N.; Mukai, T. Effect of aluminum or zinc solute addition on enhancing impact fracture toughness in Mg-Ca alloys. Acta Mater. 2016, 104, 283–294. [Google Scholar] [CrossRef]
- Lu, S.; Hu, Q.M.; Punkkinen, M.P.J.; Johansson, B.; Vitos, L. First-principles study of fcc-Ag/bcc-Fe interfaces. Phys. Rev. B 2013, 87, 224104. [Google Scholar] [CrossRef] [Green Version]
- Sinnott, S.B.; Dickey, E.C. Ceramic/metal interface structures and their relationship to atomic- and meso-scale properties. Mater. Sci. Eng. R Rep. 2003, 43, 1–59. [Google Scholar] [CrossRef]
- Yan, H.; Chen, R.; Zheng, N.; Luo, J.; Kamado, S.; Han, E. Effects of trace Gd concentration on texture and mechanical properties of hot-rolled Mg–2Zn–xGd sheets. J. Magnes. Alloy. 2013, 1, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Al-Samman, T.; Li, X. Sheet texture modification in magnesium-based alloys by selective rare earth alloying. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Processing 2011, 528, 3809–3822. [Google Scholar] [CrossRef]
- Rycroft, C.H.; Grest, G.S.; Landry, J.W.; Bazant, M.Z. Analysis of granular flow in a pebble-bed nuclear reactor. Phys. Rev. E 2006, 74, 021306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazar, E.A.; Han, J.; Srolovitz, D.J. Topological framework for local structure analysis in condensed matter. Proc. Natl. Acad. Sci. USA 2015, 112, E5769–E5776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahjoub, R.; Xu, W.; Gun, B.; Laws, K.; Kong, L.; Li, J.; Ferry, M. Heterogeneous nucleation at inoculant particles in a glass forming alloy: An ab initio molecular dynamics investigation of interfacial properties and local chemical bonding. Comput. Mater. Sci. 2015, 108, 94–102. [Google Scholar] [CrossRef]
- Deringer, V.L.; Tchougreeff, A.L.; Dronskowski, R. Crystal Orbital Hamilton Population (COHP) Analysis As Projected from Plane-Wave Basis Sets. J. Phys. Chem. A 2011, 115, 5461–5466. [Google Scholar] [CrossRef]













| Grain Number | Boundary Plane and Parallel Directions | Axis and Angle of Rotation (Three Digit Indices) | Equivalent Four Digit Rotation Axis If Rational | |
|---|---|---|---|---|
| Boundary A | Grain A1 | 58.31° | tilt | |
| Grain A2 | ||||
| Boundary B | Grain B1 | 43.37° | irrational | |
| Grain B2 | ||||
| Boundary C | Grain C1 | 61.59° | twist | |
| Grain C2 | ||||
| Boundary D | Grain D1 | 18.79° | tilt | |
| Grain D2 | ||||
| Boundary E | Grain E1 | 10.9° | twist | |
| Grain E2 | ||||
| Boundary F | Grain F1 | 74.8° < 1, −0.4, −0.25> | irrational | |
| Grain F2 | ||||
| Boundary | Cell Dimension (Å) | Number of Atoms in Simulation | Number of Atoms at GB | Solute Conc. (1 Atom) | Solute Conc. (5 Atom) | Additional Simulation |
|---|---|---|---|---|---|---|
| Boundary A | × 99.51 | 128 | 8 | 12.5% | 62.5% | none |
| Boundary B | 52.96 | 536 | 38 | 2.6% | 13.2% | 2 solute atoms = 5.3% conc. |
| Boundary C | 32.10 | 977 | 140 | 0.7% | 3.6% | 7 solute atoms = 5.0% conc. |
| Boundary D | 49.43 | 890 | 106 | 0.9% | 4.7% | None |
| Boundary E | 46.54 | 742 | 106 | 0.9% | 4.7% | None |
| Boundary F | × 43.33 | 846 | 148 | 0.7% | 3.4% | 7 solute atoms = 5.0% conc. |
| Boundary | ϒGB (J/m2) | WSEP (J/m2) | CN | Strain Width (%) |
|---|---|---|---|---|
| Boundary A | 0.584 | 1.049 | 8.4 | 3.0 |
| Boundary B | 0.205 | 1.208 | 5.1 | 4.2 |
| Boundary C | 0.445 | 1.117 | 5.6 | 7.9 |
| Boundary D | 0.465 | 1.025 | 7.3 | 6.6 |
| Boundary E | 0.379 | 0.904 | 8.1 | 2.7 |
| Boundary F | 0.427 | 0.982 | 7.6 | 7.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahjoub, R.; Stanford, N. The Energetics and Topology of Grain Boundaries in Magnesium: An Ab Initio Study. Alloys 2022, 1, 15-30. https://doi.org/10.3390/alloys1010003
Mahjoub R, Stanford N. The Energetics and Topology of Grain Boundaries in Magnesium: An Ab Initio Study. Alloys. 2022; 1(1):15-30. https://doi.org/10.3390/alloys1010003
Chicago/Turabian StyleMahjoub, Reza, and Nikki Stanford. 2022. "The Energetics and Topology of Grain Boundaries in Magnesium: An Ab Initio Study" Alloys 1, no. 1: 15-30. https://doi.org/10.3390/alloys1010003