Experimental and Simulation Studies for Purification and Etherification of Glycerol from the Biodiesel Industry
Abstract
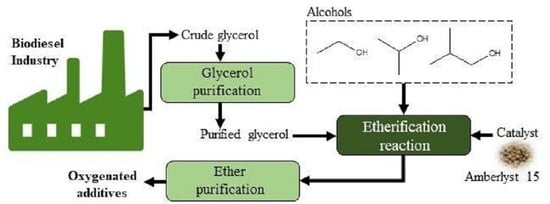
:1. Introduction
2. Experimental and Simulation Section
2.1. Materials
2.2. Physicochemical and Compositional Characterization
2.3. Purification Process for Crude Glycerol
2.3.1. Acidification and Neutralization
2.3.2. Salt Precipitation and Removal of Contaminants
2.4. Experimental Procedures for the Etherification Reaction
2.5. Purification of Products
2.6. Process Simulation
3. Results and Discussions
3.1. Physicochemical and Compositional Properties of Purified Glycerol
3.2. Etherification Reactions
3.3. Chemical Characterization of the Etherification Products
3.4. Process Simulation Using DWSIM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pramanik, T.; Tripathi, S. Biodiesel: Clean fuel of the future: New processing technologies uncover means to produce low-sulfur biomass-based diesel. Hydrocarb. Process. 2005, 84, 49–54. [Google Scholar]
- Knothe, G.; Van Gerpen, J.; Krahl, J. The Biodiesel Handbook; Elsevier: Champaign, IL, USA, 2005. [Google Scholar]
- Bournay, L.; Casanave, D.; Delfort, B.; Hillion, G.; Chodorge, J.A. New heterogeneous process for biodiesel production: A way to improve the quality and the value of the crude glycerin produced by biodiesel plants. Catal. Today 2005, 106, 190–192. [Google Scholar] [CrossRef]
- Bueno, A.V.; Pereira, M.P.; Pontes, J.V.O.; Luna, F.M.; Cavalcante, C.L., Jr. Performance and emissions characteristics of castor oil biodiesel fuel blends. Appl. Therm. Eng. 2017, 125, 559–566. [Google Scholar] [CrossRef]
- Melero, J.A.; Vicente, G.; Paniagua, M.; Morales, G.; Muñoz, P. Etherification of biodiesel-derived glycerol with ethanol for fuel formulation over sulfonic modified catalysts. Bioresour. Technol. 2012, 103, 142–151. [Google Scholar] [CrossRef]
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; da Silva César, A. Glycerol from biodiesel production: Technological paths for sustainability. Renew. Sust. Energ. Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Rodrigues, A.; Bordado, J.C.; Santos, R.G. Upgrading the glycerol from biodiesel production as a source of energy carriers and chemicals—A technological review for three chemical pathways. Energies 2017, 10, 1817. [Google Scholar] [CrossRef]
- Veluturla, S.; Archna, N.; Subba Rao, D.; Hezil, N.; Indraja, I.S.; Spoorthi, S. Catalytic valorization of raw glycerol derived from biodiesel: A review. Biofuels 2016, 9, 305–314. [Google Scholar] [CrossRef]
- Bagheri, S.; Julkapli, N.M.; Yehye, W.A. Catalytic conversion of biodiesel derived raw glycerol to value added products. Renew. Sustain. Energy Rev. 2015, 41, 113–127. [Google Scholar] [CrossRef]
- Tan, H.W.; Abdul Aziz, A.R.; Aroua, M.K. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Quispe, C.A.G.; Coronado, C.J.R.; Carvalho, J.A., Jr. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Anuar, M.R.; Abdullah, A.Z. Challenges in biodiesel industry with regards to feedstock, environmental, social and sustainability issues: A critical review. Renew. Sustain. Energy Rev. 2016, 58, 208–223. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Ghorbani, B.; Abedi, H. Biodiesel production integrated with glycerol steam reforming process, solid oxide fuel cell (SOFC) power plant. Energ. Convers. Manag. 2020, 206, 112467. [Google Scholar] [CrossRef]
- Danish, M.; Mumtaz, M.W.; Fakhar, M.; Rashid, U. Response surface methodology based optimized purification of the residual glycerol from biodiesel production process. Chiang Mai J. Sci. 2017, 44, 1570–1582. [Google Scholar]
- Chol, C.G.; Dhabhai, R.; Dalai, A.K.; Reaney, M. Purification of crude glycerol derived from biodiesel production process: Experimental studies and techno-economic analyses. Fuel Process. Technol. 2018, 178, 78–87. [Google Scholar] [CrossRef]
- Pal, P.; Chaurasia, S.P.; Upadhyaya, S.; Agarwal, M.; Sridhar, S. Glycerol purification using membrane technology. In Membrane Processes: Pervaporation, Vapor Permeation and Membrane Distillation for Industrial Scale Separations; Scrivener Publishing LLC: Beverly, MA, USA, 2018; pp. 431–463. [Google Scholar] [CrossRef]
- Poly, S.S.; Jamil, M.A.; Touchy, A.S.; Yasumura, S.; Siddiki, S.H.; Toyao, T.; Maeno, Z.; Shimizu, K.I. Acetalization of glycerol with ketones and aldehydes catalyzed by high silica Hβ zeolite. Mol. Catal. 2019, 479, 110608. [Google Scholar] [CrossRef]
- Mazarío, J.; Concepción, P.; Ventura, M.; Domine, M.E. Continuous catalytic process for the selective dehydration of glycerol over Cu-based mixed oxide. J. Catal. 2020, 385, 160–175. [Google Scholar] [CrossRef]
- Arcanjo, M.R.A.; Silva, I.J.; Rodríguez-Castellón, E.; Infantes-Molina, A.; Vieira, R.S. Conversion of glycerol into lactic acid using Pd or Pt supported on carbon as catalyst. Catal. Today 2017, 279, 317–326. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, X.; Zhao, H.; Hou, Z. Esterification of glycerol with acetic acid over SO3H—functionalized phenolic resin. Fuel 2019, 255, 111542. [Google Scholar] [CrossRef]
- Lemos, C.O.T.; Rade, L.L.; Barrozo, M.A.S.; Cardozo-Filho, L.; Hori, C.E. Study of glycerol etherification with ethanol in fixed bed reactor under high pressure. Fuel Process. Technol. 2018, 178, 1–6. [Google Scholar] [CrossRef]
- Izquierdo, J.F.; Montiel, M.; Palés, I.; Outon, P.R.; Galán, M.; Jutglar, L.; Villarrubia, M.; Izquierdo, M.P.; Hermo, M.P.; Ariza, X. Fuel additives from glycerol etherification with light olefins: State of the art. Renew. Sustain. Energy Rev. 2012, 16, 6717–6724. [Google Scholar] [CrossRef]
- Bozkurt, Ö.D.; Tunç, F.M.; Bağlar Çelebi, N.S.; Günbaş, İ.D.; Uzun, A. Alternative fuel additives from glycerol by etherification with isobutene: Structure–performance relationships in solid catalysts. Fuel Process. Technol. 2015, 138, 780–804. [Google Scholar] [CrossRef]
- Pariente, S.; Tanchoux, N.; Fajula, F. Etherification of glycerol with ethanol over solid acid catalyst. Green Chem. 2009, 11, 1256–1261. [Google Scholar] [CrossRef]
- Yuan, Z.; Xia, S.; Chen, P.; Hou, Z.; Zheng, X. Etherification of biodiesel-based glycerol with bioethanol over tungstophosphoric acid to synthesize glyceryl ethers. Energ. Fuel 2011, 25, 3186–3191. [Google Scholar] [CrossRef]
- Pinto, B.P.; Lyra, J.T.; Nascimento, J.A.C.; Mota, C.J.A. Ethers of glycerol and ethanol as bioadditives for biodiesel. Fuel 2016, 168, 76–80. [Google Scholar] [CrossRef]
- Mravec, D.; Turan, A.; Filková, A.; Mikesková, N.; Volkovicsová, E.; Onyestyák, G.; Harnos, S.; Lónyi, F.; Valyon, J.; Kaszonyi, A. Catalytic etherification of bioglycerol with bioethanol over H-Beta, H-Y and H-MOR zeolites. Fuel Process. Technol. 2017, 159, 111–117. [Google Scholar] [CrossRef]
- Veiga, P.M.; Gomes, A.C.L.; Veloso, C.O.; Henriques, C.A. Acid zeolites for glycerol etherification with ethyl alcohol: Catalytic activity and catalyst properties. Appl. Catal. A-Gen. 2017, 548, 2–15. [Google Scholar] [CrossRef]
- Pinzi, S.; Garcia, I.L.; Lopez-Gimenez, F.J.; Castro, M.D.L.; Dorado, G.; Dorado, M.P. The ideal vegetable oil-based biodiesel composition: A review of social, economical and technical implications. Energy Fuel 2009, 23, 2325–2341. [Google Scholar] [CrossRef]
- Tangsriwong, K.; Lapchit, P.; Kittijungjit, T.; Klamrassamee, T.; Sukjai, Y.; Laoonual, Y. Modeling of chemical processes using commercial and open-source software: A comparison between Aspen Plus and DWSIM. IOP Conf. Ser. Earth Environ. Sci. 2020, 463, 012057. [Google Scholar]
- Medeiros, D. DWSIM—Open Source Process Simulator. Available online: http://sourceforge.net/projects/dwsim (accessed on 15 May 2023).
- Almeida, L.A.; Vilas Bôas, R.N.; Mendes, M.F. Process simulation of biodiesel production from vegetable oil deodorization distillate using hydrotalcite-hydroxyapatite as catalyst. Res. Soc. Dev. 2021, 10, e15210615452. [Google Scholar] [CrossRef]
- Da Cruz, D.M.B.; da Silva, C.M.C.B.; de Souza Menezes, J.D.; Magalhães, A.M.C.; de Faro, F.S. Optimization of biodiesel and glycerol production process from palm oil and soy by modeling in DWSIM software. Braz. J. Dev. 2021, 7, 77121–77145. [Google Scholar] [CrossRef]
- Cortes-Peña, Y.; Kumar, D.; Singh, V.; Guest, J.S. BioSTEAM: A Fast and Flexible Platform for the Design, Simulation, and Techno-Economic Analysis of Biorefineries under Uncertainty. ACS Sustain. Chem. Eng. 2020, 8, 3302–3310. [Google Scholar] [CrossRef]
- Hassan, M. A simulation of energy generation from Jatropha solid residues in a power plant in Jazan city, KSA. Heliyon 2022, 8, e09352. [Google Scholar] [CrossRef]
- Parente, E.J.S., Jr.; de Oliveira, L.B.; Luna, F.M.T.; Cavalcante, C.L., Jr. Integrated production of biolubricants and biodiesel: Process simulation and technical–economic analysis. Biomass Convers. Biorefin. 2023, 13, 1–24. [Google Scholar] [CrossRef]
- Canakci, M.; Sanli, H. Biodiesel production from various feedstocks and their effects on the fuel properties. J. Ind. Microbiol. Biot. 2008, 35, 431–441. [Google Scholar] [CrossRef] [PubMed]
- ASTM D891; Standard Test Methods for Specific Gravity, Apparent, of Liquid Industrial Chemicals. Book of Standards, Volume: 06.04; ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- ASTM D1293; Standard Test Methods for pH of Water. Book of Standards Volume: 11.01; ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- ASTM D1125; Standard Test Methods for Electrical Conductivity and Resistivity of Water. Book of Standards Volume: 11.01; ASTM International: West Conshohocken, PA, USA, 2014. [CrossRef]
- ISO 2098; Glycerols for Industrial Use—Determination of Ash—Gravimetric Method. International Organization for Standardization: Geneva, Switzerland, 1972.
- AOCS EA 6-94; Determination of Crude Glycerin, Titrimetric Method, AOCS Official Method. American Oil Chemists’ Society: Urbana, IL, USA, 2009.
- Nanda, M.R.; Yuan, Z.; Qin, W.; Poirier, M.A.; Chunbao, X. Purification of crude glycerol using acidification: Effects of acid types and product characterization. Austin J. Chem. Eng. 2014, 1, 1004. [Google Scholar]
- Ferreira, M.O.; Sousa, M.E.B.D.; Pereira, C.G. Purification of crude glycerine obtained from transesterification of cottonseed oil. Int. J. Chem. React. Eng. 2013, 11, 385–392. [Google Scholar] [CrossRef]
- Hutchison, B.R.M.; Wallace, J.S. Influence of fuel volatility on particulate matter emissions from a production DISI engine. Fuel 2021, 303, 121206. [Google Scholar] [CrossRef]
- Dobrowolski, A.; Mituła, P.; Rymowicz, W.; Mirończuk, A.M. Efficient conversion of crude glycerol from various industrial wastes into single cell oil by yeast Yarrowia lipolytica. Bioresour. Technol. 2016, 207, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Escribà, M.; Eras, J.; Villorbina, G.; Balcells, M.; Blanch, C.; Barniol, N.; Canela, R. Use of Crude Glycerol from Biodiesel Producers and Fatty Materials to Prepare Allyl Esters. Waste Biomass Valoriz. 2011, 2, 285–290. [Google Scholar] [CrossRef]
- Indran, V.P.; Saud, A.S.H.; Maniam, G.P.; Taufiq-Yap, Y.H.; Rahim, M.H.B. Viable Glycerol Carbonate Synthesis Through Direct Crude Glycerol Utilization from Biodiesel Industry. Waste Biomass Valoriz. 2017, 8, 1049–1059. [Google Scholar] [CrossRef]
- Sittijunda, S.; Reungsang, A. Methane Production from the Co-digestion of Algal Biomass with Crude Glycerol by Anaerobic Mixed Cultures. Waste Biomass Valoriz. 2020, 11, 1873–1881. [Google Scholar] [CrossRef]









| Properties | Results (Mean ± SD) | References [43,46,47,48,49] |
|---|---|---|
| Glycerol content (% wt.) | 46.0 ± 0.3 | 12–81 |
| Alkalinity (mL.N/g) | 94.0 ± 0.5 | 56.0–110.0 |
| Conductivity (µS/cm) | 1,894 ± 10 | - |
| Density (g/cm3) at 20 °C | 1.00 ± 0.07 | 0.9–1.05 |
| Refractive index (at 20 °C) | 1.43 ± 0.20 | - |
| pH | 10.1 ± 0.5 | 4.5–10.5 |
| Ash content (% wt.) | 3.7 ± 0.2 | 3.0–6.0 |
| Properties | Purified Glycerol (Mean ± SD) | Commercial Glycerol (Mean ± SD) | Methods |
|---|---|---|---|
| Glycerol content (% wt.) | 98.99 ±0.50 | 99.50 ± 0.40 | AOCS EA6-94 |
| Alkalinity (ml.N/g) | 0.05 ± 0.02 | 0.05 ± 0.01 | IUPAC/ACD 1980 |
| Conductivity (µS/cm) | 0.31 ± 0.06 | 0.42 ± 0.05 | ASTM D1125 |
| Density (g/cm3) at 20 °C | 1.25 ± 0.05 | 1.26 ± 0.02 | ASTM D891 |
| Refractive index (at 20 °C) | 1.47 ± 0.20 | 1.47 ± 0.01 | ASTM D1747 |
| pH | 7.2 ± 0.5 | 8.0 ± 0.1 | ASTM D1293 |
| Ash content (% wt.) | 0.09 ± 0.05 | 0.06 ± 0.01 | ISO 2098 |
| Experimental Conditions | Results (% wt.) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Alcohol | Molar Ratio (Glycerol/Alcohol) | XG (%) | SME (%) | ηME (%) | SDE (%) | ηDE (%) | STE (%) | ηTE (%) | ηTotal (%) |
| Ethanol | 1:3 | 78.3 | 31.8 | 24.9 | 35.3 | 27.6 | 32.7 | 25.6 | 78.1 |
| Ethanol | 1:6 | 83.4 | 37.2 | 31.0 | 62.7 | 52.3 | 0.0 | 0.0 | 83.3 |
| Ethanol | 1:12 | 97.5 | 19.5 | 19.0 | 37.6 | 36.7 | 42.8 | 41.7 | 97.4 |
| Isopropanol | 1:3 | 56.0 | 97.1 | 54.4 | 2.8 | 1.6 | 0.2 | 0.1 | 56.1 |
| Isopropanol | 1:6 | 79.8 | 97.6 | 77.9 | 2.4 | 1.9 | 0.0 | 0.0 | 79.8 |
| Isopropanol | 1:12 | 85.2 | 97.1 | 82.7 | 2.8 | 2.4 | 0.0 | 0.0 | 85.1 |
| Conditions | Input | Output | ||
|---|---|---|---|---|
| STR-04 | STR-06 | Methanol | Glycerol | |
| Temperature (°C) | 60.0 | 60.0 | 64.8 | 45.3 |
| Pressure (bar) | 1.01 | 1.01 | 1.01 | 1.01 |
| Mass Flow (kg/h) | 222.81 | 10.00 | 116.97 | 840.63 |
| Volumetric Flow (m3/h) | 0.26 | 0.00 | 0.16 | 1.07 |
| Specific Enthalpy (kJ/kg) | −936.31 | 570.49 | −1041.42 | −722.99 |
| Component mole fraction | STR-04 | STR-06 | Methanol | Glycerol |
| Methanol | 0.729 | 0.000 | 0.990 | 0.000 |
| Catalysts and salts | 0.052 | 1.000 | 0.000 | 0.000 |
| Glycerol | 0.219 | 0.000 | 0.010 | 1.000 |
| Etherification with Ethanol (1:3 Molar Ratio) | ||||
|---|---|---|---|---|
| Conditions | Input | Output | ||
| Glycerol | Ethanol | STR-12 | STR-13 | |
| Temperature (°C) | 229.2 | 25.0 | 110.0 | 110.0 |
| Pressure (bar) | 1.01 | 1.01 | 1.01 | 1.01 |
| Mass Flow (kg/h) | 95.51 | 143.32 | 59.62 | 179.21 |
| Volumetric Flow (m3/h) | 0.09 | 0.18 | 51.91 | 0.20 |
| Spec. Enthalpy (kJ/kg) | −477.09 | −922.33 | 137.42 | −614.17 |
| Component mole fraction | ||||
| Ethanol | 0.000 | 1.000 | 0.636 | 0.160 |
| Glycerol | 1.000 | 0.000 | 0.000 | 0.091 |
| 3-ethoxypropan-1,2-diol | 0.000 | 0.000 | 0.000 | 0.095 |
| 1,3-diethoxypropan-2-ol | 0.000 | 0.000 | 0.000 | 0.114 |
| 1,2,3-triethoxypropane | 0.000 | 0.000 | 0.000 | 0.112 |
| Water | 0.000 | 0.000 | 0.361 | 0.425 |
| Etherification with Isopropanol (1:3 Molar Ratio) | ||||
|---|---|---|---|---|
| Conditions | Input | Output | ||
| Glycerol | Isoprop. | STR-12 | STR-13 | |
| Temperature (°C) | 229.2 | 25.0 | 110.0 | 110.0 |
| Pressure (bar) | 1.01 | 1.01 | 1.01 | 1.01 |
| Mass Flow (kg/h) | 95.51 | 745.13 | 675.91 | 164.72 |
| Volumetric Flow (m3/h) | 0.09 | 0.95 | 371.02 | 0.19 |
| Spec. Enthalpy (kJ/kg) | −477.09 | −754.49 | 140.44 | −587.04 |
| Component mole fraction | ||||
| Isopropanol | 0.000 | 1.000 | 0.932 | 0.335 |
| Glycerol | 1.000 | 0.000 | 0.000 | 0.127 |
| 3-ethoxypropan-1,2-diol | 0.000 | 0.000 | 0.000 | 0.494 |
| 1,3-diethoxypropan-2-ol | 0.000 | 0.000 | 0.000 | 0.011 |
| Water | 0.000 | 0.000 | 0.067 | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, S.S.O.; Nascimento, M.R.; Lima, R.J.P.; Luna, F.M.T.; Cavalcante Júnior, C.L. Experimental and Simulation Studies for Purification and Etherification of Glycerol from the Biodiesel Industry. AppliedChem 2023, 3, 492-508. https://doi.org/10.3390/appliedchem3040031
Silva SSO, Nascimento MR, Lima RJP, Luna FMT, Cavalcante Júnior CL. Experimental and Simulation Studies for Purification and Etherification of Glycerol from the Biodiesel Industry. AppliedChem. 2023; 3(4):492-508. https://doi.org/10.3390/appliedchem3040031
Chicago/Turabian StyleSilva, Silvia S. O., Matheus R. Nascimento, Ricardo J. P. Lima, Francisco Murilo Tavares Luna, and Célio Loureiro Cavalcante Júnior. 2023. "Experimental and Simulation Studies for Purification and Etherification of Glycerol from the Biodiesel Industry" AppliedChem 3, no. 4: 492-508. https://doi.org/10.3390/appliedchem3040031