Updating the Phylogeography and Temporal Evolution of Mitochondrial DNA Haplogroup U8 with Special Mention to the Basques
Abstract
:1. Introduction
2. Material and Methods
2.1. Samples
2.2. Sequence Classification
2.3. Match-Based Distances between Populations
2.4. Other Statistical Analyses
2.5. Coalescence Age Estimations
3. Results
3.1. U8 Phylogeography of Present-Day Populations
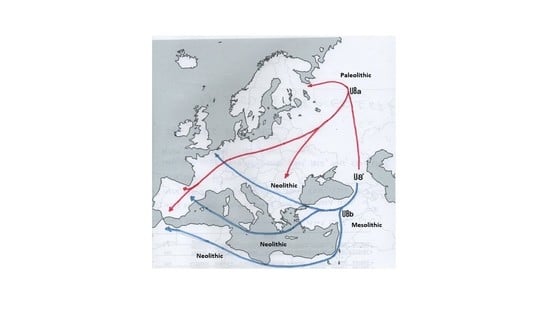
3.2. U8 Phylogeography in the Past
3.3. Haplogroup U8 Coalescent Age Estimates
4. Discussion
4.1. The Extinction of mtDNA Lineages
4.2. The Peopling of Europe from an U8a Perspective
4.3. The Peopling of Europe from a U8b Perspective
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maca-Meyer, N.; González, A.M.; Larruga, J.M.; Flores, C.; Cabrera, V.M. Major genomic mitochondrial lineages delineate early human expansions. BMC Genet. 2001, 2, 13. [Google Scholar] [CrossRef] [Green Version]
- Larruga, J.M.; Marrero, P.; Abu-Amero, K.K.; Golubenko, M.V.; Cabrera, V.M. Carriers of mitochondrial DNA macrohaplogroup R colonized Eurasia and Australasia from a southeast Asia core area. BMC Evol. Biol. 2017, 17, 115. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, T.; Laitinen, V.; Salmela, E.; Andersen, P.; Huoponen, K.; Savontaus, M.-L.; Lahermo, P. Migration waves to the Baltic Sea region. Ann. Hum. Genet. 2008, 72, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Lunkina, A.V.; Denisova, G.A.; Derenko, M.V.; Malarchuk, B.A. Mitochondrial DNA variation in two Russian populations from Novgorod oblast. Genetika 2004, 40, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Varesi, L.; Memmi, M.; Cristofari, M.-C.; Mameli, G.; Calo, C.; Vona, G. Mitochondrial control-region sequence variation in the Corsican population, France. Am. J. Hum. Biol. Off. J. Hum. Biol. Assoc. 2000, 12, 339–351. [Google Scholar] [CrossRef]
- Cherni, L.; Loueslati, B.Y.; Pereira, L.; Ennafaa, H.; Amorim, A.; El Gaaied, A.B.A. Female gene pools of Berber and Arab neighboring communities in central Tunisia: Microstructure of mtDNA variation in North Africa. Hum. Biol. 2005, 77, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Ermini, L.; Olivieri, C.; Rizzi, E.; Corti, G.; Bonnal, R.; Soares, P.; Luciani, S.; Marota, I.; De Bellis, G.; Richards, M.B.; et al. Complete mitochondrial genome sequence of the Tyrolean Iceman. Curr. Biol. 2008, 18, 1687–1693. [Google Scholar] [CrossRef]
- Richards, M.; Macaulay, V.; Hickey, E.; Vega, E.; Sykes, B.; Guida, V.; Rengo, C.; Sellitto, D.; Cruciani, F.; Kivisild, T.; et al. Tracing European founder lineages in the Near Eastern mtDNA pool. Am. J. Hum. Genet. 2000, 67, 1251–1276. [Google Scholar] [CrossRef]
- Costa, M.D.; Pereira, J.B.; Pala, M.; Fernandes, V.; Olivieri, A.; Achilli, A.; Perego, U.A.; Rychkov, S.; Naumova, O.; Hatina, J.; et al. A substantial prehistoric European ancestry amongst Ashkenazi maternal lineages. Nat. Commun. 2013, 4, 2543. [Google Scholar] [CrossRef] [Green Version]
- Isern, N.; Fort, J.; de Rioja, V.L. The ancient cline of haplogroup K implies that the Neolithic transition in Europe was mainly demic. Sci. Rep. 2017, 7, 11229. [Google Scholar] [CrossRef]
- Fu, Q.; Posth, C.; Hajdinjak, M.; Petr, M.; Mallick, S.; Fernandes, D.; Furtwängler, A.; Haak, W.; Meyer, M.; Mittnik, A.; et al. The genetic history of ice age Europe. Nature 2016, 534, 200–205. [Google Scholar] [CrossRef] [Green Version]
- Posth, C.; Renaud, G.; Mittnik, A.; Drucker, D.G.; Rougier, H.; Cupillard, C.; Valentin, F.; Thevenet, C.; Furtwängler, A.; Wißing, C.; et al. Pleistocene mitochondrial genomes suggest a single major dispersal of non-Africans and a Late Glacial population turnover in Europe. Curr. Biol. 2016, 26, 827–833. [Google Scholar] [CrossRef] [Green Version]
- González, A.M.; García, O.; Larruga, J.M.; Cabrera, V.M. The mitochondrial lineage U8a reveals a Paleolithic settlement in the Basque country. BMC Genom. 2006, 7, 124. [Google Scholar] [CrossRef] [Green Version]
- Weissensteiner, H.; Pacher, D.; Kloss-Brandstätter, A.; Forer, L.; Specht, G.; Bandelt, H.-J.; Kronenberg, F.; Salas, A.; Schönherr, S. HaploGrep 2: Mitochondrial haplogroup classification in the era of high-throughput sequencing. Nucleic Acids Res. 2016, 44, W58–W63. [Google Scholar] [CrossRef]
- Van Oven, M.; Kayser, M. Updated comprehensive phylogenetic tree of global human mitochondrial DNA variation. Hum. Mutat. 2009, 30, E386–E394. [Google Scholar] [CrossRef]
- Saillard, J.; Forster, P.; Lynnerup, N.; Bandelt, H.J.; Nørby, S. mtDNA variation among Greenland Eskimos: The edge of the Beringian expansion. Am. J. Hum. Genet. 2000, 67, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Soares, P.; Ermini, L.; Thomson, N.; Mormina, M.; Rito, T.; Röhl, A.; Salas, A.; Oppenheimer, S.; Macaulay, V.; Richards, M.B. Correcting for purifying selection: An improved human mitochondrial molecular clock. Am. J. Hum. Genet. 2009, 84, 740–759. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, V.M. Counterbalancing the time-dependent effect on the human mitochondrial DNA molecular clock. BMC Evol. Biol. 2020, 20, 78. [Google Scholar] [CrossRef]
- Cabrera, V.M. Human molecular evolutionary rate, time dependency and transient polymorphism effects viewed through ancient and modern mitochondrial DNA genomes. Sci. Rep. 2021, 11, 5036. [Google Scholar] [CrossRef]
- Zaidi, A.A.; Wilton, P.R.; Su, M.S.-W.; Paul, I.M.; Arbeithuber, B.; Anthony, K.; Nekrutenko, A.; Nielsen, R.; Makova, K.D. Bottleneck and selection in the germline and maternal age influence transmission of mitochondrial DNA in human pedigrees. Proc. Natl. Acad. Sci. USA 2019, 116, 25172–25178. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Mittnik, A.; Johnson, P.L.; Bos, K.; Lari, M.; Bollongino, R.; Sun, C.; Giemsch, L.; Schmitz, R.; Burger, J.; et al. A revised timescale for human evolution based on ancient mitochondrial genomes. Curr. Biol. 2013, 23, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Behar, D.M.; van Oven, M.; Rosset, S.; Metspalu, M.; Loogväli, E.-L.; Silva, N.M.; Kivisild, T.; Torroni, A.; Villems, R. A “Copernican” reassessment of the human mitochondrial DNA tree from its root. Am. J. Hum. Genet. 2012, 90, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Modi, A.; Vai, S.; Posth, C.; Vergata, C.; Zaro, V.; Diroma, M.A.; Boschin, F.; Capecchi, G.; Ricci, S.; Ronchitelli, A.; et al. More data on ancient human mitogenome variability in Italy: New mitochondrial genome sequences from three Upper Palaeolithic burials. Ann. Hum. Biol. 2021, 48, 213–222. [Google Scholar] [CrossRef]
- Ho, S.Y.; Shapiro, B. Skyline-plot methods for estimating demographic history from nucleotide sequences. Mol. Ecol. Resour. 2011, 11, 423–434. [Google Scholar] [CrossRef]
- Prüfer, K.; Posth, C.; Yu, H.; Stoessel, A.; Spyrou, M.A.; Deviese, T.; Mattonai, M.; Ribechini, E.; Higham, T.; Velemínský, P.; et al. A genome sequence from a modern human skull over 45,000 years old from Zlatý kůň in Czechia. Nat. Ecol. Evol. 2021, 5, 820–825. [Google Scholar] [CrossRef]
- Hublin, J.-J.; Sirakov, N.; Aldeias, V.; Bailey, S.; Bard, E.; Delvigne, V.; Endarova, E.; Fagault, Y.; Fewlass, H.; Hajdinjak, M.; et al. Initial Upper Palaeolithic Homo sapiens from Bacho Kiro Cave, Bulgaria. Nature 2020, 581, 299–302. [Google Scholar] [CrossRef]
- Raghavan, M.; Skoglund, P.; Graf, K.E.; Metspalu, M.; Albrechtsen, A.; Moltke, I.; Rasmussen, S.; Stafford, T.W., Jr.; Orlando, L.; Metspalu, E.; et al. Upper Palaeolithic Siberian genome reveals dual ancestry of Native Americans. Nature 2014, 505, 87–91. [Google Scholar] [CrossRef]
- Sikora, M.; Seguin-Orlando, A.; Sousa, V.C.; Albrechtsen, A.; Korneliussen, T.; Ko, A.; Rasmussen, S.; Dupanloup, I.; Nigst, P.R.; Bosch, M.D.; et al. Ancient genomes show social and reproductive behavior of early Upper Paleolithic foragers. Science 2017, 358, 659–662. [Google Scholar] [CrossRef] [Green Version]
- Dubut, V.; Chollet, L.; Murail, P.; Cartault, F.; Béraud-Colomb, E.; Serre, M.; Mogentale-Profizi, N. mtDNA polymorphisms in five French groups: Importance of regional sampling. Eur. J. Hum. Genet. 2004, 12, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Lazaridis, I.; Nadel, D.; Rollefson, G.; Merrett, D.C.; Rohland, N.; Mallick, S.; Fernandes, D.; Novak, M.; Gamarra, B.; Sirak, K.; et al. Genomic insights into the origin of farming in the ancient Near East. Nature 2016, 536, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Mathieson, I.; Alpaslan-Roodenberg, S.; Posth, C.; Szécsényi-Nagy, A.; Rohland, N.; Mallick, S.; Olalde, I.; Broomandkhoshbacht, N.; Candilio, F.; Cheronet, O.; et al. The genomic history of southeastern Europe. Nature 2018, 555, 197–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieson, I.; Lazaridis, I.; Rohland, N.; Mallick, S.; Patterson, N.; Roodenberg, S.A.; Harney, E.; Stewardson, K.; Fernandes, D.; Novak, M.; et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature 2015, 528, 499–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, E.R.; Gonzalez-Fortes, G.; Connell, S.; Siska, V.; Eriksson, A.; Martiniano, R.; McLaughlin, R.L.; Llorente, M.G.; Cassidy, L.M.; Gamba, C.; et al. Upper Palaeolithic genomes reveal deep roots of modern Eurasians. Nat. Commun. 2015, 6, 8912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margaryan, A.; Derenko, M.; Hovhannisyan, H.; Malyarchuk, B.; Heller, R.; Khachatryan, Z.; Avetisyan, P.; Badalyan, R.; Bobokhyan, A.; Melikyan, V.; et al. Eight millennia of matrilineal genetic continuity in the south Caucasus. Curr. Biol. 2017, 27, 2023–2028. [Google Scholar] [CrossRef] [Green Version]
- Schönberg, A.; Theunert, C.; Li, M.; Stoneking, M.; Nasidze, I. High-throughput sequencing of complete human mtDNA genomes from the Caucasus and West Asia: High diversity and demographic inferences. Eur. J. Hum. Genet. 2011, 19, 988–994. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.-X.; Yan, S.; Qin, Z.-D.; Wang, Y.; Tan, J.-Z.; Li, H.; Jin, L. Major population expansion of East Asians began before neolithic time: Evidence of mtDNA genomes. PLoS ONE 2011, 6, e25835. [Google Scholar] [CrossRef]
- Feldman, M.; Fernández-Domínguez, E.; Reynolds, L.; Baird, D.; Pearson, J.; Hershkovitz, I.; May, H.; Goring-Morris, N.; Benz, M.; Gresky, J.; et al. Late Pleistocene human genome suggests a local origin for the first farmers of central Anatolia. Nat. Commun. 2019, 10, 1218. [Google Scholar] [CrossRef] [Green Version]
- Hofmanová, Z.; Kreutzer, S.; Hellenthal, G.; Sell, C.; Diekmann, Y.; Díez-del-Molino, D.; Van Dorp, L.; López, S.; Kousathanas, A.; Link, V.; et al. Early farmers from across Europe directly descended from Neolithic Aegeans. Proc. Natl. Acad. Sci. USA 2016, 113, 6886–6891. [Google Scholar] [CrossRef] [Green Version]
- Lipson, M.; Szécsényi-Nagy, A.; Mallick, S.; Pósa, A.; Stégmár, B.; Keerl, V.; Rohland, N.; Stewardson, K.; Ferry, M.; Michel, M.; et al. Parallel palaeogenomic transects reveal complex genetic history of early European farmers. Nature 2017, 551, 368–372. [Google Scholar] [CrossRef]
- Olalde, I.; Schroeder, H.; Sandoval-Velasco, M.; Vinner, L.; Lobón, I.; Ramirez, O.; Civit, S.; García Borja, P.; Salazar-García, D.C.; Talamo, S.; et al. A common genetic origin for early farmers from Mediterranean Cardial and Central European LBK cultures. Mol. Biol. Evol. 2015, 32, 3132–3142. [Google Scholar] [CrossRef] [Green Version]
- Malyarchuk, B.; Derenko, M.; Grzybowski, T.; Perkova, M.; Rogalla, U.; Vanecek, T.; Tsybovsky, I. The peopling of Europe from the mitochondrial haplogroup U5 perspective. PLoS ONE 2010, 5, e10285. [Google Scholar] [CrossRef] [Green Version]
- Malyarchuk, B.; Grzybowski, T.; Derenko, M.; Perkova, M.; Vanecek, T.; Lazur, J.; Gomolcák, P.; Tsybovsky, I. Mitochondrial DNA phylogeny in eastern and western Slavs. Mol. Biol. Evol. 2008, 25, 1651–1658. [Google Scholar] [CrossRef]
- Derenko, M.; Malyarchuk, B.; Denisova, G.; Perkova, M.; Litvinov, A.; Grzybowski, T.; Dambueva, I.; Skonieczna, K.; Rogalla, U.; Tsybovsky, I.; et al. Western Eurasian ancestry in modern Siberians based on mitogenomic data. BMC Evol. Biol. 2014, 14, 217. [Google Scholar] [CrossRef] [Green Version]
- Kivisild, T.; Reidla, M.; Metspalu, E.; Rosa, A.; Brehm, A.; Pennarun, E.; Parik, J.; Geberhiwot, T.; Usanga, E.; Villems, R. Ethiopian mitochondrial DNA heritage: Tracking gene flow across and around the gate of tears. Am. J. Hum. Genet. 2004, 75, 752–770. [Google Scholar] [CrossRef] [Green Version]
- Haak, W.; Forster, P.; Bramanti, B.; Matsumura, S.; Brandt, G.; Tänzer, M.; Villems, R.; Renfrew, C.; Gronenborn, D.; Alt, K.W.; et al. Ancient DNA from the first European farmers in 7500-year-old Neolithic sites. Science 2005, 310, 1016–1018. [Google Scholar] [CrossRef] [Green Version]
- Haak, W.; Balanovsky, O.; Sanchez, J.J.; Koshel, S.; Zaporozhchenko, V.; Adler, C.J.; Der Sarkissian, C.S.I.; Brandt, G.; Schwarz, C.; Nicklisch, N.; et al. Ancient DNA from European early neolithic farmers reveals their near eastern affinities. PLoS Biol. 2010, 8, e1000536. [Google Scholar] [CrossRef] [Green Version]



| Region | Sample (Ref.) | U8a | U8b | U8 | U8a/U8b Ratio |
|---|---|---|---|---|---|
| Iberia (IB) | 9704 (Table S1) | 23 (0.24) | 20 (0.21) | 43 (0.44) | 1.15 |
| Italy (IT) | 5049 (Table S2) | 6 (0.12) | 16 (0.32) | 22 (0.44) | 0.375 |
| Balkans (BK) | 3762 (Table S3) | 1 (0.03) | 6 (0.16) | 7 (0.19) | 0.167 |
| Turkey (ME) | 818 (Table S9) | 0 | 2 (0.24) | 2 (0.24) | 0.0 |
| Basque Country (BQ) | 1985 (Table S1) | 10 (0.50) | 2 (0.10) | 12 (0.60) | 5.0 |
| France (WE) | 2397 (Table S5) | 13 (0.54) | 1 (0.04) | 14 (0.58) | 13.0 |
| British Islands (WE) | 327,665 (Table S5) | 655 (0.20) | 131 (0.04) | 786 (0.24) | 5.0 |
| Central Europe (CE) | 2891 (Table S4) | 6 (0.21) | 3 (0.10) | 9 (0.31) | 0.334 |
| Northern Europe (NE) | 8166 (Table S7) | 61 (0.75) | 3 (0.04) | 64 (0.79) | 20.3 |
| Eastern Europe (EE) | 22,112 (Table S6) | 100 (0.45) | 29 (0.13) | 129 (0.58) | 3.45 |
| Caucasus (CU) | 4183 (Table S8) | 2 (0.05) | 23 (0.55) | 25 (0.60) | 0.087 |
| Middle East (ME) | 13,000 (Table S10) | 2 (0.01) | 55 (0.42) | 57 (0.44) | 0.036 |
| South Asia (SA) | 12,753 (Table S12) | 1 (0.008) | 3 (0.023) | 4 (0.031) | 0.333 |
| Central Asia (CA) | 9421 (Table S11) | 4 (0.04) | 7 (0.07) | 11 (0.12) | 0.571 |
| Northern Africa (NA) | 5145 (Table S13) | 2 (0.04) | 18 (0.35) | 20 (0.39) | 0.111 |
| Period | Date | Sample (Table S14) | U8a | U8b | U8c | U8 |
|---|---|---|---|---|---|---|
| Paleolithic | (2 My–12,000 BCE) | 55 | 8 (14.5) | 0 | 4 (7.3) | 12 (21.8) |
| Mesolithic | (12,000–8300 BCE) | 300 | 0 | 3 (1.0) | 0 | 3 (1.0) |
| Neolithic | (8300–4500 BCE) | 2078 | 4 (0.19) | 27 (1.30) | 0 | 31 (1.49) |
| Chalcolithic | (4500–3300 BCE) | 936 | 1 (0.11) | 6 (0.64) | 0 | 7 (0.75) |
| Bronze | (3300–1200 BCE) | 1701 | 1 (0.06) | 12 (0.71) | 0 | 13 (0.77) |
| Iron/Historic | (1200–present) | 2669 | 5 (0.19) | 15 (0.56) | 0 | 20 (0.75) |
| Lineages | Haplogroup | Years per Mutation | Mean | 95% CI |
|---|---|---|---|---|
| Mal’ta 1 | U* | 2454 | 46,391 | 32,769–60,013 |
| Cioclovina 1 | U* | 2454 | 32,435 | 21,275–44,043 |
| U2′3′4′7′8′9 | U* | 2454 | 45,155 | 31,717–58,593 |
| Paleolithic | U2′3′4′7′8′9 | 2454 | 42,691 | 37,447–47,934 |
| Paleolithic | U8c | 2454 | 33,443 | 31,222–35,666 |
| U8c | U8* | 2454 | 43,259 | 31,115–55,404 |
| Extant (1) | U8* | 3624 | 53,092 | 38,532–67,652 |
| Extant + 0.05 interval (2) | U8* | 3205 | 73,330 | 56,242–90,418 |
| Extant | U8* | Time dependent | 42,062 | 29,101–55,023 |
| Extant | U8a | 3624 | 29,898 | 18,981–40,815 |
| Extant + 0.05 interval | U8a | 3205 | 33,332 | 21,843–44,821 |
| Extant | U8a | Time dependent | 25,994 | 15,796–36,192 |
| Extant | U8b | 3624 | 43,995 | 30,729–57,261 |
| Extant + 0.05 interval | U8b | 3205 | 68,131 | 51,639–84,623 |
| Extant | U8b | Time dependent | 39,088 | 26,592–51,578 |
| Clade | Age in Years ± 2 se | Connected Regions |
|---|---|---|
| U8a2 | 12,322 ± 7014 | Iberia–Basque–Britain |
| U8a1a + 15,903 | 10,872 ± 6573 | Eastern Europe–northern Europe |
| U8a1a1a + 16,146! | 9060 ± 6020 | Basque–Britain–northern Europe |
| U8a1b | 7248 ± 5367 | Britain–northern Europe |
| U8a1a1b | 6741 ± 5177 | Eastern Europe |
| U8a1a3 | 6342 ± 5010 | Western Europe–England |
| U8a1a1a2 | 6342 ± 5036 | Eastern Europe |
| U8a1a4 | 6052 ± 4920 | Basque–northern Europe |
| U8a1a2 | 6052 ± 4899 | Central Europe–Britain |
| U8a1a1 + 6380 | 6052 ± 4900 | Western Europe–northern Europe |
| U8a1a1b1 | 4059 ± 4050 | Eastern Europe–northern Europe |
| U8a1a1a1 | 3624 ± 3704 | Central Europe |
| Clade | Age in Years ± 2 se | Connected Regions |
|---|---|---|
| U8b1a2 + 14,364, 16,259 | 36,240 ± 12,033 | Middle East |
| U8b1b2 | 20,536 ± 8967 | Caucasus–Middle East–Balkans |
| U8b1a1 + 10,454 | 18,120 ± 8508 | Caucasus–Middle East–Italy |
| U8b1a2b | 17,033 ± 8246 | Caucasus–M.East–Balkans–E.Europe–N.Europe–N.Africa |
| U8b1b1 + 9947 | 15,704 ± 7745 | Caucasus–northern Africa |
| U8b1b + 146, 3432, 13,155 | 15,221 ± 7771 | Central Asia–eastern Europe–Italy |
| U8b1a1 + 10,454,12,308! | 13,046 ± 7211 | Middle East–eastern Europe |
| U8b1a1 + 6515G,10,632 | 11,959 ± 6809 | Middle East–northern Europe |
| U8b1b + 7705,14,323,16,290 | 8154 ± 5692 | Central Europe–Britain–Iberia |
| U8b1b1 + 16,094 | 5177 ± 4494 | Eastern Europe–Italy–C.Europe–N.Europe |
| U8b1b1 (X16,094, 9947) | 4918 ± 4050 | Middle East–E.Europe–C.Europe–Italy–Iberia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera, V.M. Updating the Phylogeography and Temporal Evolution of Mitochondrial DNA Haplogroup U8 with Special Mention to the Basques. DNA 2022, 2, 104-115. https://doi.org/10.3390/dna2020008
Cabrera VM. Updating the Phylogeography and Temporal Evolution of Mitochondrial DNA Haplogroup U8 with Special Mention to the Basques. DNA. 2022; 2(2):104-115. https://doi.org/10.3390/dna2020008
Chicago/Turabian StyleCabrera, Vicente M. 2022. "Updating the Phylogeography and Temporal Evolution of Mitochondrial DNA Haplogroup U8 with Special Mention to the Basques" DNA 2, no. 2: 104-115. https://doi.org/10.3390/dna2020008