Cycloadditions and Cyclization Reactions via Post-Synthetic Modification and/or One-Pot Methodologies for the Stabilization of Imine-Based Covalent Organic Frameworks
Definition
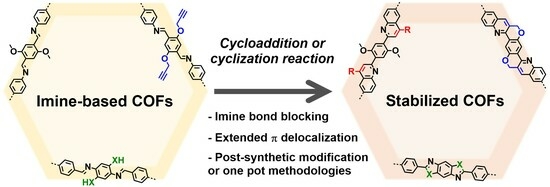
:1. Introduction
2. Cycloadditions and Cyclization Reactions for the Stabilization of Imine-Based Covalent Organic Frameworks
2.1. Formal [4 + 2] Cycloadditions: The Povarov Reaction

2.2. Other Reactions for the Synthesis of Quinoline-Based COFs
2.3. Intramolecular Oxidative Cyclization Reactions
3. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.-Y.; Wang, T. Covalent Organic Frameworks in Catalytic Organic Synthesis. Adv. Synth. Catal. 2021, 363, 144–193. [Google Scholar] [CrossRef]
- Chen, H.; Jena, H.S.; Feng, X.; Leus, K.; Van Der Voort, P. Engineering Covalent Organic Frameworks as Heterogeneous Photocatalysts for Organic Transformations. Angew. Chem. Int. Ed. 2022, 61, e202204938. [Google Scholar] [CrossRef]
- Alonso-Navarro, M.J.; Barrio, J.; Royuela, S.; Karjule, N.; Ramos, M.M.; Martínez, J.I.; Shalom, M.; Segura, J.L. Photocatalytic Degradation of Organic Pollutants through Conjugated Poly(Azomethine) Networks Based on Terthiophene-Naphthalimide Assemblies. RSC Adv. 2021, 11, 2701–2705. [Google Scholar] [CrossRef]
- Royuela, S.; Martínez-Periñán, E.; Arrieta, M.P.; Martínez, J.I.; Ramos, M.M.; Zamora, F.; Lorenzo, E.; Segura, J.L. Oxygen Reduction Using a Metal-Free Naphthalene Diimide-Based Covalent Organic Framework Electrocatalyst. Chem. Commun. 2020, 56, 1267–1270. [Google Scholar] [CrossRef]
- Martínez-Fernández, M.; Martínez-Periñán, E.; Royuela, S.; Martínez, J.I.; Zamora, F.; Lorenzo, E.; Segura, J.L. Covalent Organic Frameworks Based on Electroactive Naphthalenediimide as Active Electrocatalysts toward Oxygen Reduction Reaction. Appl. Mater. Today 2022, 26, 101384. [Google Scholar] [CrossRef]
- Martínez-Fernández, M.; Martínez-Periñán, E.; Martínez, J.I.; Gordo-Lozano, M.; Zamora, F.; Segura, J.L.; Lorenzo, E. Evaluation of the Oxygen Reduction Reaction Electrocatalytic Activity of Postsynthetically Modified Covalent Organic Frameworks. ACS Sustain. Chem. Eng. 2023, 11, 1763–1773. [Google Scholar] [CrossRef]
- Luo, R.; Yang, Y.; Chen, K.; Liu, X.; Chen, M.; Xu, W.; Liu, B.; Ji, H.; Fang, Y. Tailored Covalent Organic Frameworks for Simultaneously Capturing and Converting CO2 into Cyclic Carbonates. J. Mater. Chem. A 2021, 9, 20941–20956. [Google Scholar] [CrossRef]
- Bian, G.; Yin, J.; Zhu, J. Recent Advances on Conductive 2D Covalent Organic Frameworks. Small 2021, 17, 2006043. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Ahmed, N.; Amjad, M.W.; Hussain, M.A.; Elsherif, M.A.; Ejaz, H.; Alotaibi, N.H. Covalent Organic Frameworks (COFs) as Multi-Target Multifunctional Frameworks. Polymers 2023, 15, 267. [Google Scholar] [CrossRef]
- Royuela, S.; García-Garrido, E.; Martín Arroyo, M.; Mancheño, M.J.; Ramos, M.M.; González-Rodríguez, D.; Somoza, Á.; Zamora, F.; Segura, J.L. Uracil Grafted Imine-Based Covalent Organic Framework for Nucleobase Recognition. Chem. Commun. 2018, 54, 8729–8732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Feng, J.; Lian, Y.; Sun, X.; Wang, M.; Sun, M. Advances of the Functionalized Covalent Organic Frameworks for Sample Preparation in Food Field. Food Chem. 2023, 405, 134818. [Google Scholar] [CrossRef]
- Tran, Q.N.; Lee, H.J.; Tran, N. Covalent Organic Frameworks: From Structures to Applications. Polymers 2023, 15, 1279. [Google Scholar] [CrossRef]
- Royuela, S.; Almarza, J.; Mancheño, M.J.; Pérez-Flores, J.C.; Michel, E.G.; Ramos, M.M.; Zamora, F.; Ocón, P.; Segura, J.L. Synergistic Effect of Covalent Bonding and Physical Encapsulation of Sulfur in the Pores of a Microporous COF to Improve Cycling Performance in Li-S Batteries. Chem. A Eur. J. 2019, 25, 12394–12404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Fernández, M.; Gavara, R.; Royuela, S.; Fernández-Ecija, L.; Martínez, J.I.; Zamora, F.; Segura, J.L. Following the Light: 3D-Printed COF@poly(2-hydroxyethyl Methacrylate) Dual Emissive Composite with Response to Polarity and Acidity. J. Mater. Chem. A 2022, 10, 4634–4643. [Google Scholar] [CrossRef]
- Segura, J.L.; Mancheño, M.J.; Zamora, F. Covalent Organic Frameworks Based on Schiff-Base Chemistry: Synthesis, Properties and Potential Applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef]
- Qian, C.; Feng, L.; Teo, W.L.; Liu, J.; Zhou, W.; Wang, D.; Zhao, Y. Imine and Imine-Derived Linkages in Two-Dimensional Covalent Organic Frameworks. Nat. Rev. Chem. 2022, 6, 881–898. [Google Scholar] [CrossRef]
- Jiang, G.; Zou, W.; Ou, Z.; Zhang, W.; Liang, Z.; Du, L. Stabilization of 2D Imine-linked Covalent Organic Frameworks: From Linkage Chemistry to Interlayer Interaction. Chem. Eur. J. 2022, 29, e202203610. [Google Scholar] [CrossRef]
- Grunenberg, L.; Savasci, G.; Terban, M.W.; Duppel, V.; Moudrakovski, I.; Etter, M.; Dinnebier, R.E.; Ochsenfeld, C.; Lotsch, B.V. Amine-Linked Covalent Organic Frameworks as a Platform for Postsynthetic Structure Interconversion and Pore-Wall Modification. J. Am. Chem. Soc. 2021, 143, 3430–3438. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, H.; Xiao, Y.; Ren, S.; Wang, Y.; Li, L. Efficient Exfoliation of Covalent Organic Frameworks by a Facile Thiol-Ene Reaction. Chem. Eng. J. 2023, 454, 140283. [Google Scholar] [CrossRef]
- Li, X.-T.; Zou, J.; Wang, T.-H.; Ma, H.-C.; Chen, G.-J.; Dong, Y.-B. Construction of Covalent Organic Frameworks via Three-Component One-Pot Strecker and Povarov Reactions. J. Am. Chem. Soc. 2020, 142, 6521–6526. [Google Scholar] [CrossRef] [PubMed]
- Segura, J.L.; Royuela, S.; Mar Ramos, M. Post-Synthetic Modification of Covalent Organic Frameworks. Chem. Soc. Rev. 2019, 48, 3903–3945. [Google Scholar] [CrossRef] [PubMed]
- Acharjya, A.; Pachfule, P.; Roeser, J.; Schmitt, F.J.; Thomas, A. Vinylene-Linked Covalent Organic Frameworks by Base-Catalyzed Aldol Condensation. Angew. Chem. Int. Ed. 2019, 58, 14865–14870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jadhav, T.; Fang, Y.; Liu, C.-H.; Dadvand, A.; Hamzehpoor, E.; Patterson, W.; Jonderian, A.; Stein, R.S.; Perepichka, D.F. Transformation between 2D and 3D Covalent Organic Frameworks via Reversible [2 + 2] Cycloaddition. J. Am. Chem. Soc. 2020, 142, 8862–8870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira de Paiva, W.; de Freitas Rego, Y.; de Fátima, Â.; Fernandes, S.A. The Povarov Reaction: A Versatile Method to Synthesize Tetrahydroquinolines, Quinolines and Julolidines. Synthesis 2022, 54, 3162–3179. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Cai, S.; Lei, X.; Altoe, V.; Hong, F.; Urban, J.J.; Ciston, J.; Chan, E.M.; Liu, Y. Facile Transformation of Imine Covalent Organic Frameworks into Ultrastable Crystalline Porous Aromatic Frameworks. Nat. Commun. 2018, 9, 2998. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Raya, J.; Richard, F.; Baaziz, W.; Ersen, O.; Ciesielski, A.; Samorì, P. Synthesis of Robust MOFs@COFs Porous Hybrid Materials via an Aza-Diels–Alder Reaction: Towards High-Performance Supercapacitor Materials. Angew. Chem. Int. Ed. 2020, 59, 19602–19609. [Google Scholar] [CrossRef]
- Zhao, Y.; Sui, Z.; Chang, Z.; Wang, S.; Liang, Y.; Liu, X.; Feng, L.; Chen, Q.; Wang, N. A Trifluoromethyl-Grafted Ultra-Stable Fluorescent Covalent Organic Framework for Adsorption and Detection of Pesticides. J. Mater. Chem. A 2020, 8, 25156–25164. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y.; Jia, X.; Zhang, Q.; Mao, J.; Feng, Y.; Yin, D.; Zhao, W.; Zhang, Y.; Ouyang, G.; et al. An Ultrastable 2D Covalent Organic Framework Coating for Headspace Solid-Phase Microextraction of Organochlorine Pesticides in Environmental Water. J. Hazard. Mater. 2023, 452, 131228. [Google Scholar] [CrossRef]
- Chang, Z.; Liang, Y.; Wang, S.; Qiu, L.; Lu, Y.; Feng, L.; Sui, Z.; Chen, Q. A Novel Fluorescent Covalent Organic Framework Containing Boric Acid Groups for Selective Capture and Sensing of Cis-Diol Molecules. Nanoscale 2020, 12, 23748–23755. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Li, Y.; Xia, M.; Xia, T.; Sun, H.; Sui, Z.; Hu, X.-M.; Chen, Q. Ultra-Stable Fluorescent 2D Covalent Organic Framework for Rapid Adsorption and Selective Detection of Radioiodine. Microporous Mesoporous Mater. 2021, 319, 111046. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, Y.; Wu, Z.; Li, C.; Fan, R.; Feng, L.; Wang, W.; Chen, Q. A Novel Fluorescent Covalent Organic Framework for the Selective Detection of Fluoride Ion. J. Mater. Sci. 2022, 57, 13425–13432. [Google Scholar] [CrossRef]
- Xiao, Z.; Nie, X.; Li, Y.; Nie, Y.; Lu, L.; Tian, X. Boric Acid Functional Fluorescent Covalent-Organic Framework for Sensitive and Selective Visualization of CH3Hg+. ACS Appl. Mater. Interfaces 2023, 15, 9524–9532. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, Y.; Xia, T.; Tian, H.; Li, Y.; Sui, Z.; Yuan, N.; Tian, X.; Chen, Q. Pyrimidine-Functionalized Covalent Organic Framework and Its Cobalt Complex as an Efficient Electrocatalyst for Oxygen Evolution Reaction. ChemSusChem 2021, 14, 4556–4562. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Luo, J.; Liang, Y.; Yu, X.; Zhao, Y.; Li, Y.; Wang, W.; Sui, Z.; Tian, X.; Chen, Q. Tetrazole Functionalized Benzoquinoline-Linked Covalent Organic Frameworks with Efficient Performance for Electrocatalytic H2O2 Production and Li-S Batteries. Mater. Chem. Front. 2023, 7, 1650–1658. [Google Scholar] [CrossRef]
- Liu, X.; Xia, M.; Zhao, Y.; Xia, T.; Li, Y.; Xiao, J.; Sui, Z.; Chen, Q. Cationic Covalent Organic Framework via Cycloaddition Reactions as Sulfur-Loaded Matrix for Lithium-Sulfur Batteries. Mater. Today Chem. 2022, 23, 100664. [Google Scholar] [CrossRef]
- Liang, Y.; Xia, M.; Zhao, Y.; Wang, D.; Li, Y.; Sui, Z.; Xiao, J.; Chen, Q. Functionalized Triazine-Based Covalent Organic Frameworks Containing Quinoline via Aza-Diels-Alder Reaction for Enhanced Lithium-Sulfur Batteries Performance. J. Colloid Interface Sci. 2022, 608, 652–661. [Google Scholar] [CrossRef]
- Ren, X.R.; Bai, B.; Zhang, Q.; Hao, Q.; Guo, Y.; Wan, L.J.; Wang, D. Constructing Stable Chromenoquinoline-Based Covalent Organic Frameworks via Intramolecular Povarov Reaction. J. Am. Chem. Soc. 2022, 144, 2488–2494. [Google Scholar] [CrossRef]
- Lyu, H.; Li, H.; Hanikel, N.; Wang, K.; Yaghi, O.M. Covalent Organic Frameworks for Carbon Dioxide Capture from Air. J. Am. Chem. Soc. 2022, 144, 12989–12995. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, J.; Yu, Z.; Cong, M.; Wang, Y.; Wang, M.; Li, G.; Li, Z.; Zhao, Y. Post-Synthetic Fully π-Conjugated Three-Dimensional Covalent Organic Frameworks for High-Performance Lithium Storage. ACS Appl. Mater. Interfaces 2023, 15, 830–837. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, J.; Ren, Z.; Guan, Z.H. Postsynthetically Modified Hydrophobic Covalent Organic Frameworks for Enhanced Oil/Water and CH4/C2H2 Separation. Chem. Eng. J. 2022, 448, 137687. [Google Scholar] [CrossRef]
- Hayashi, Y. Pot Economy and One-Pot Synthesis. Chem. Sci. 2016, 7, 866–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, B.J.; Wu, W.X.; Ding, L.G.; Dong, Y.B. Sulfonic Acid and Ionic Liquid Functionalized Covalent Organic Framework for Efficient Catalysis of the Biginelli Reaction. J. Org. Chem. 2021, 86, 3024–3032. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Han, Y.; Zheng, X.; Zhao, J.; Man, Y.; Sun, L.; Zhao, Y. One-Pot Synthesis of Fully-Conjugated Chemically Stable Two-Dimensional Covalent Organic Framework. Chin. J. Chem. 2022, 40, 699–704. [Google Scholar] [CrossRef]
- Chen, H.; Liu, W.; Liu, C.; Sun, J.; Bourda, L.; Morent, R.; De Geyter, N.; Van Deun, R.; Van Hecke, K.; Leus, K.; et al. Two in One: A Brønsted Acid Grafted Photoactive Covalent Organic Framework as Metal-Free Dual Photocatalyst for Aerobic Oxidative C-C Cleavage. Appl. Catal. B Environ. 2022, 319, 121920. [Google Scholar] [CrossRef]
- Ding, L.G.; Yao, B.J.; Wu, W.X.; Yu, Z.G.; Wang, X.Y.; Kan, J.L.; Dong, Y.B. Metalloporphyrin and Ionic Liquid-Functionalized Covalent Organic Frameworks for Catalytic CO2Cycloaddition via Visible-Light-Induced Photothermal Conversion. Inorg. Chem. 2021, 60, 12591–12601. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Y.J.; Ma, S.H.; Yang, C.; Wang, Z.P.; Ding, S.Y.; Li, Y.; Wang, W. Fused-Ring-Linked Covalent Organic Frameworks. J. Am. Chem. Soc. 2022, 144, 6594–6603. [Google Scholar] [CrossRef]
- Zhao, X.; Pang, H.; Huang, D.; Liu, G.; Hu, J.; Xiang, Y. Construction of Ultrastable Nonsubstituted Quinoline-Bridged Covalent Organic Frameworks via Rhodium-Catalyzed Dehydrogenative Annulation. Angew. Chem. Int. Ed. 2022, 61, e202208833. [Google Scholar] [CrossRef]
- Pang, H.; Huang, D.; Zhu, Y.; Zhao, X.; Xiang, Y. One-Pot Cascade Construction of Nonsubstituted Quinoline-Bridged Covalent Organic Frameworks. Chem. Sci. 2023, 14, 1543–1550. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, L.; Chu, T.; Niu, H.; Wang, J.; Cai, Y. Constructing Chemical Stable 4-Carboxyl-Quinoline Linked Covalent Organic Frameworks via Doebner Reaction for Nanofiltration. Nat. Commun. 2022, 13, 2615. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Pan, Q.; Wu, C.; Hao, W.; Xu, J.; Chen, R.; Liu, J.; Li, Z.; Zhao, Y. Construction of Fully Conjugated Covalent Organic Frameworks via Facile Linkage Conversion for Efficient Photoenzymatic Catalysis. J. Am. Chem. Soc. 2020, 142, 5958–5963. [Google Scholar] [CrossRef] [PubMed]
- Ranjeesh, K.C.; Illathvalappil, R.; Veer, S.D.; Peter, J.; Wakchaure, V.C.; Goudappagouda; Raj, K.V.; Kurungot, S.; Babu, S.S. Imidazole-Linked Crystalline Two-Dimensional Polymer with Ultrahigh Proton-Conductivity. J. Am. Chem. Soc. 2019, 141, 14950–14954. [Google Scholar] [CrossRef] [PubMed]
- Waller, P.J.; Alfaraj, Y.S.; Diercks, C.S.; Jarenwattananon, N.N.; Yaghi, O.M. Conversion of Imine to Oxazole and Thiazole Linkages in Covalent Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 9099–9103. [Google Scholar] [CrossRef]
- Haase, F.; Troschke, E.; Savasci, G.; Banerjee, T.; Duppel, V.; Dörfler, S.; Grundei, M.M.J.; Burow, A.M.; Ochsenfeld, C.; Kaskel, S.; et al. Topochemical Conversion of an Imine- into a Thiazole-Linked Covalent Organic Framework Enabling Real Structure Analysis. Nat. Commun. 2018, 9, 2600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Niu, H.; Zhao, W.; Xu, L.; Zhang, H.; Cai, Y. Ultrafine Pd Nanoparticles Loaded Benzothiazole-Linked Covalent Organic Framework for Efficient Photocatalytic C-C Cross-Coupling Reactions. RSC Adv. 2020, 10, 29402–29407. [Google Scholar] [CrossRef]
- Seo, J.-M.; Noh, H.-J.; Jeong, H.Y.; Baek, J.-B. Converting Unstable Imine-Linked Network into Stable Aromatic Benzoxazole-Linked One via Post-Oxidative Cyclization. J. Am. Chem. Soc. 2019, 141, 11786–11790. [Google Scholar] [CrossRef]
- Kaur, M.; Kumar, R. A Minireview on the Scope of Cadogan Cyclization Reactions Leading to Diverse Azaheterocycles. Asian J. Org. Chem. 2022, 11, e202200092. [Google Scholar] [CrossRef]
- Yang, S.; Yang, C.; Dun, C.; Mao, H.; Khoo, R.S.H.; Klivansky, L.M.; Reimer, J.A.; Urban, J.J.; Zhang, J.; Liu, Y. Covalent Organic Frameworks with Irreversible Linkages via Reductive Cyclization of Imines. J. Am. Chem. Soc. 2022, 144, 9827–9835. [Google Scholar] [CrossRef]
- Wei, P.F.; Qi, M.Z.; Wang, Z.P.; Ding, S.Y.; Yu, W.; Liu, Q.; Wang, L.K.; Wang, H.Z.; An, W.K.; Wang, W. Benzoxazole-Linked Ultrastable Covalent Organic Frameworks for Photocatalysis. J. Am. Chem. Soc. 2018, 140, 4623–4631. [Google Scholar] [CrossRef]
- Wu, C.J.; Li, X.Y.; Li, T.R.; Shao, M.Z.; Niu, L.J.; Lu, X.F.; Kan, J.L.; Geng, Y.; Dong, Y. Bin Natural Sunlight Photocatalytic Synthesis of Benzoxazole-Bridged Covalent Organic Framework for Photocatalysis. J. Am. Chem. Soc. 2022, 144, 18750–18755. [Google Scholar] [CrossRef]
- Cui, W.R.; Zhang, C.R.; Xu, R.H.; Chen, X.R.; Yan, R.H.; Jiang, W.; Liang, R.P.; Qiu, J.D. Low Band Gap Benzoxazole-Linked Covalent Organic Frameworks for Photo-Enhanced Targeted Uranium Recovery. Small 2021, 17, 2006882. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.R.; Zhang, C.R.; Liang, R.P.; Liu, J.; Qiu, J.D. Covalent Organic Framework Sponges for Efficient Solar Desalination and Selective Uranium Recovery. ACS Appl. Mater. Interfaces 2021, 13, 31561–31568. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jia, Z.; Bai, Y.; Wang, X.; Hodgkiss, S.E.; Chen, L.; Chong, S.Y.; Wang, X.; Yang, H.; Xu, Y.; et al. Synthesis of Stable Thiazole-Linked Covalent Organic Frameworks via a Multicomponent Reaction. J. Am. Chem. Soc. 2020, 142, 11131–11138. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Chandra Shit, S.; Mandal, H.; Rabeah, J.; Kashyap, S.S.; Nailwal, Y.; Shinde, D.B.; Lai, Z.; Mondal, J. Benzothiazole-Linked Metal-Free Covalent Organic Framework Nanostructures for Visible-Light-Driven Photocatalytic Conversion of Phenylboronic Acids to Phenols. ACS Appl. Nano Mater. 2021, 4, 11732–11742. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gala, E.; Ramos, M.M.; Segura, J.L. Cycloadditions and Cyclization Reactions via Post-Synthetic Modification and/or One-Pot Methodologies for the Stabilization of Imine-Based Covalent Organic Frameworks. Encyclopedia 2023, 3, 795-807. https://doi.org/10.3390/encyclopedia3030057
Gala E, Ramos MM, Segura JL. Cycloadditions and Cyclization Reactions via Post-Synthetic Modification and/or One-Pot Methodologies for the Stabilization of Imine-Based Covalent Organic Frameworks. Encyclopedia. 2023; 3(3):795-807. https://doi.org/10.3390/encyclopedia3030057
Chicago/Turabian StyleGala, Elena, M. Mar Ramos, and José L. Segura. 2023. "Cycloadditions and Cyclization Reactions via Post-Synthetic Modification and/or One-Pot Methodologies for the Stabilization of Imine-Based Covalent Organic Frameworks" Encyclopedia 3, no. 3: 795-807. https://doi.org/10.3390/encyclopedia3030057