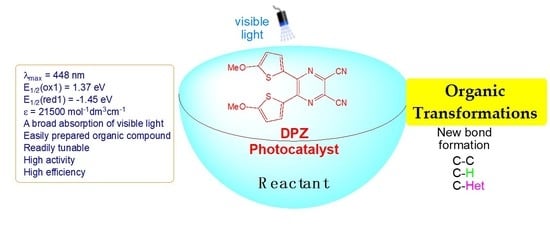
Recent Advances of Dicyanopyrazine (DPZ) in Photoredox Catalysis
Abstract
:1. Introduction
2. Synthesis of DPZ
3. Photochemistry of DPZ
4. Applications in Photoredox Chemistry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Narayanam, J.M.R.; Stephenson, C.R.J. Visible light photoredox catalysis: Applications in organic synthesis. Chem. Soc. Rev. 2011, 40, 102–113. [Google Scholar] [CrossRef]
- Schultz, D.M.; Yoon, T.P. Solar Synthesis: Prospects in Visible Light Photocatalysis. Science 2014, 343, 1239176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasu, Y.; Koike, T.; Akita, M. Visible-light-induced synthesis of a variety of trifluoromethylated alkenes from potassium vinyltrifluoroborates by photoredox catalysis. Chem. Commun. 2013, 49, 2037–2638. [Google Scholar] [CrossRef]
- Chatterjee, T.; Roh, G.; Shoaib, M.A.; Suhl, C.H.; Kim, J.S.; Cho, C.G.; Cho, E.J. Visible-Light-Induced Synthesis of Carbazoles by in Situ Formation of Photosensitizing Intermediate. Org. Lett. 2017, 19, 1906–1909. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Wang, D.H.; Chen, B.; Zhong, J.J.; Tung, C.H.; Wu, L.Z. Visible Light-Induced Synthesis of 3,4-Diarylthiophenes from 3,4-Diaryl-2,5-dihydrothiophenes. J. Org. Chem. 2012, 77, 6773–6777. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, F.; Yue, H.; Zhao, X.; Li, J.; Wei, W. Photocatalyst-Free Visible Light-Induced Synthesis of β-Oxo Sulfones via Oxysulfonylation of Alkenes with Arylazo Sulfones and Dioxygen in Air. Adv. Synth. Catal. 2019, 361, 5277–5282. [Google Scholar] [CrossRef]
- Li, Z.; Song, H.; Guo, R.; Zuo, M.; Hou, C.; Sun, S.; He, X.; Sun, Z.; Chu, W. Visible-light-induced condensation cyclization to synthesize benzimidazoles using fluorescein as a photocatalyst. Green Chem. 2019, 21, 3602–3605. [Google Scholar] [CrossRef]
- Xuan, J.; Xiao, W.J. Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2012, 51, 6828–6838. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ma, M.; Gong, X.; Fan, X.; Walsh, P.J. Reductive Cross-Coupling of Aldehydes and Imines Mediated by Visible Light Photoredox Catalysis. Org. Lett. 2019, 21, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.F.; Zhang, G.W.; Zhu, S.L. Visible-light-induced synthesis of benzothiophenes and benzoselenophenes via the annulation of thiophenols or 1,2-diphenyldiselane with alkynes. Tetrahedron 2017, 73, 2727–2730. [Google Scholar] [CrossRef] [Green Version]
- Marzo, L.; Pagire, S.K.; Reiser, O.; König, B. Visible-Light Photocatalysis: Does it make a difference in Organic Synthesis? Angew. Chem. Int. Ed. 2018, 57, 10034–10072. [Google Scholar] [CrossRef]
- Shaw, M.H.; Twilton, J.; MacMillan, D.W.C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6926. [Google Scholar] [CrossRef] [PubMed]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.N.; Lei, Z.; Ngai, M.Y. β-Selective Reductive Coupling of Alkenylpyridines with Aldehydes and Imines via Synergistic Lewis Acid/Photoredox Catalysis. J. Am. Chem. Soc. 2017, 139, 5003–5006. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, D.C.; Castro, L.; Churcher, I.; Rees, D.C.; Thomas, A.W.; Wilson, D.M.; Wood, A. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 2018, 10, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.J.; Sevrin, M.J.; Stephenson, C.R.J. Visible Light Photocatalysis: Applications and New Disconnections in the Synthesis of Pharmaceutical Agents. Org. Process Res. Dev. 2016, 20, 1134–1147. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Zhao, G.; Busch, E.; Wang, T. Metal-free photocatalytic thiol–ene/thiol–yne reactions. Org. Biomol. Chem. 2019, 17, 1955–1961. [Google Scholar] [CrossRef]
- Fan, W.; Yang, Q.; Xu, F.; Li, P. A Visible-Light-Promoted Aerobic Metal-Free C-3 Thiocyanation of Indoles. J. Org. Chem. 2014, 79, 10588–10592. [Google Scholar] [CrossRef]
- Li, X.; Gu, X.; Li, Y.; Li, P. Aerobic Transition-Metal-Free Visible-Light Photoredox Indole C-3 Formylation Reaction. ACS Catal. 2014, 4, 1897–1900. [Google Scholar] [CrossRef]
- Zhang, W.; Xiang, X.X.; Chen, J.; Yang, C.; Pan, Y.-L.; Cheng, J.-P.; Meng, Q.; Li, X. Direct C–H difluoromethylation of heterocycles via organic photoredox catalysis. Nat. Commun. 2020, 11, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, L.; Liu, X.; Guo, P.; Yu, Y.; Cao, H. Visible-Light-Induced Regioselective Dicarbonylation of Indolizines with Oxoaldehydes via Direct C–H Functionalization. Org. Lett. 2020, 22, 3841–3845. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Chen, W.; Jiang, C.; Lu, H. Visible light-induced aerobic oxidative cross-coupling reaction: Preparation of α-indolyl glycine derivatives. New J. Chem. 2020, 44, 313–316. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, C.; Li, S.; Ding, A.; Gu, G.; Guo, H. Eosin Y-catalyzed photooxidation of triarylphosphines under visible light irradiation and aerobic conditions. RSC Adv. 2017, 7, 13240–13243. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, V.; Singh, P.K.; Singh, P.P. Microwave-mediated InCl3catalysed three component efficient annelation of thiadiazinyl ring on 4-Amino-4H-[1,2,4]triazole-3-thiol. Chem. Heterocycl. Compd. 2014, 50, 573–578. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, P.K.; Singh, P.P. Novel one-pot facile synthesis of thiopyranopyrazole using[H mim]HSO4 catalyst. Croat. Chem. Acta 2014, 87, 91–95. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, P.K.; Singh, P.P. Eosin Y Catalyzed Visible-light-promoted One –Pot Facile Synthesis of 1,3,4-Thiadiazole. Croat. Chem. Acta 2015, 88, 59–65. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, P.K.; Singh, P.P. Eosin Y catalyzed visible-light-promoted one –pot facile synthesis of 2-aminobenzothiazole. Croat. Chem. Acta 2015, 88, 227–233. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, P.K.; Sinha, S.; Singh, P.P. [bmIm]OH Catalysed Four component One-pot facile Synthesis of Pyrazoloimidazole-2-thione-N-nucleosides. Rev. Roum. Chim. 2016, 61, 755–761. [Google Scholar]
- Srivastava, V.; Singh, P.K.; Singh, P.P. Trifluoromethylation of Disubstituted Morpholines by Metal-FreeVisible Light Photoredox Catalysis. Asian J. Chem. 2016, 28, 2159–2163. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, P.K.; Singh, P.P. Visible Light Promoted Allylic C–H Oxidation. Croat. Chem. Acta 2017, 90, 435–441. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, P.P. Eosin Y catalyzed photoredox synthesis: A review. RSC Adv. 2017, 7, 31377. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, P.K.; Kanaujia, S.; Singh, P.P. Photoredox catalysed synthesis of amino alcohol. New J. Chem. 2018, 42, 688. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, P.P.; Srivastava, V. Facile aerobic photo-oxidative synthesis of sulfinic ester. Croat. Chem. Acta 2018, 91, 383–387. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, P.K.; Singh, P.P. Visible light photoredoxcatalysedamidation of carboxylic acids with amines. Tetrahedron Lett. 2019, 60, 40–43. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, P.K.; Singh, P.P. Photocatalysed eosin Y mediated C(sp3)−H alkylation of amine substrates via direct HAT. Tetrahedron Lett. 2019, 60, 1333–1336. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, P.K.; Singh, P.P. Eosin Y catalysed visible-light mediated aerobic oxidation of tertiary amines. Tetrahedron Lett. 2019, 60, 151041. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, P.K.; Singh, P.P. Visible Light Promoted Synthesis of Disubstituted 1,2,3-Thiadiazoles. Rev. Roum. Chim. 2020, 65, 221–226. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, P.K.; Srivastava, A.; Singh, P.P. Recent application of visible-light induced radicals in C-S bond formation. RSC Adv. 2020, 10, 20046–20056. [Google Scholar] [CrossRef]
- Srivastava, A.; Singh, P.K.; Ali, A.; Singh, P.P.; Srivastava, V. Recent Applications of Rose Bengal catalysis in N -heterocycles: A Short Review. RSC Adv. 2020, 10, 39495. [Google Scholar] [CrossRef]
- Singh, P.P.; Srivastava, V. Recent advances of 4DPAIPN in photocatalytic transformations. Org. Biomol. Chem. 2021, 19, 313–321. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, P.K.; Srivastava, A.; Singh, P.P. Synthetic applications of flavin photocatalysis: A review. RSC Adv. 2021, 11, 14251–14259. [Google Scholar] [CrossRef]
- Bureš, F.; Cermakova, H.; Kulhanek, J.; Ludwig, M.; Kuznik, W.; Kityk, I.V.; Mikysek, T.; Ruzicka, A. Structure–Property Relationships and Nonlinear Optical Effects in Donor-Substituted Dicyanopyrazine-Derived Push–Pull Chromophores with Enlarged and Varied π-Linkers. Eur. J. Org. Chem. 2012, 2012, 529–538. [Google Scholar] [CrossRef]
- Dokladalova, L.; Bureš, F.; Kuznik, W.; Kityk, I.V.; Wojciechowski, A.; Mikysek, T.; Almonasy, N.; Ramaiyan, M.; Padelkova, Z.; Kulhanek, J.; et al. Dicyanobenzene and dicyanopyrazine derived X-shaped charge-transfer chromophores: Comparative and structure–property relationship study. Org. Biomol. Chem. 2014, 12, 5517–5527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Zhang, C.; Chin, K.F.; Pytela, O.; Wei, G.; Liu, H.; Bureš, F.; Jiang, Z. Dicyanopyrazine-derived push–pull chromophores for highly efficient photoredox catalysis. RSC Adv. 2014, 4, 30062–30067. [Google Scholar] [CrossRef] [Green Version]
- Hloušková, Z.; Klikar, M.; Pytela, O.; Almonasy, N.; Růžička, A.; Jandová, V.; Bureš, F. Structural elaboration of dicyanopyrazine: Towards push–pull molecules with tailored photoredox activity. RSC Adv. 2019, 9, 23797–23809. [Google Scholar] [CrossRef] [Green Version]
- Hloušková, Z.; Tydlitát, J.; Kong, M.; Pytela, O.; Mikysek, T.; Klikar, M.; Almonasy, N.; Dvořák, M.; Jiang, Z.; Růžička, A.; et al. Structure-Catalytic Activity in a Series of Push-Pull Dicyanopyrazine/Dicyanoimidazole Photoredox Catalysts. ChemistrySelect. 2018, 3, 4262–4270. [Google Scholar] [CrossRef]
- Vega-Peñaloza, A.; Mateos, J.; Companyó, X.; Escudero-Casao, M.; Dell’Amico, L. A Rational Approach to Organo-Photocatalysis: Novel Designs and Structure-Property Relationships. Angew. Chem. Int. Ed. 2021, 60, 1082–1097. [Google Scholar] [CrossRef]
- Qin, Y.; Zhu, L.; Luo, S. Organocatalysis in Inert C–H Bond Functionalization. Chem. Rev. 2017, 117, 13–9433. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.C. Enantioselective synthesis enabled by visible light photocatalysis. Org. Biomol. Chem. 2020, 18, 4298–4353. [Google Scholar] [CrossRef]
- Yin, Y.; Zhao, X.; Qiao, B.; Jiang, Z. Cooperative photoredox and chiral hydrogen-bonding catalysis. Org. Chem. Front. 2020, 7, 1283–1296. [Google Scholar] [CrossRef]
- Bagdi, A.K.; Rahman, M.; Bhattacherjee, D.; Zyryanov, G.V.; Ghosh, S.; Chupakhinb, O.N.; Hajra, A. Visible light promoted cross-dehydrogenative coupling: A decade update. Green Chem. 2020, 22, 6632–6681. [Google Scholar] [CrossRef]
- Ranjan, P.; Pillitteri, S.; Van der Eycken, E.V.; Sharma, U.K. Photochemical methods for deuterium labelling of organic molecules. Green Chem. 2020, 22, 7725–7736. [Google Scholar] [CrossRef]
- Lv, X.; Xu, H.; Yin, Y.; Zhao, X.; Jiang, Z. Visible Light-Driven Cooperative DPZ and Chiral Hydrogen-Bonding Catalysis. Chin. J. Chem. 2020, 38, 1480–1488. [Google Scholar] [CrossRef]
- Jiang, C.; Chen, W.; Zheng, W.H.; Lu, H. Advances in asymmetric visible-light photocatalysis, 2015–2019. Org. Biomol. Chem. 2019, 17, 8673–8689. [Google Scholar] [CrossRef]
- Zhou, R.; Ma, L.; Yanga, X.; Cao, J. Recent advances in visible-light photocatalytic deuteration reactions. Org. Chem. Front. 2021, 8, 426–444. [Google Scholar] [CrossRef]
- Sancheti, S.P.; Urvashi; Shah, M.P.; Patil, N.T. Ternary Catalysis: A stepping stone toward Multicatalysis. ACS Catal. 2020, 10, 3462–3489. [Google Scholar] [CrossRef]
- Cao, K.; Tan, S.M.; Lee, R.; Yang, S.; Jia, H.; Zhao, X.; Qiao, B.; Jiang, Z. Catalytic Enantioselective Addition of Prochiral Radicals to Vinylpyridines. J. Am. Chem. Soc. 2019, 141, 5437–5443. [Google Scholar] [CrossRef]
- Hou, M.; Lin, L.; Chai, X.; Zhao, X.; Qiao, B.; Jiang, Z. Enantioselective photoredox dehalogenative protonation. Chem. Sci. 2019, 10, 6629–6634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Liu, X.; Li, J.; Zhao, X.; Qiaoa, B.; Jiang, Z. Catalytic enantioselective radical coupling of activated ketones with N-aryl glycines. Chem. Sci. 2018, 9, 8094–8098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Liu, Y.; Chai, G.; Qiao, B.; Zhao, X.; Jiang, Z. Organocatalytic Enantioselective Addition of α-Aminoalkyl Radicals to Isoquinolines. Org. Lett. 2018, 20, 6298–6301. [Google Scholar] [CrossRef]
- Yin, Y.; Dai, Y.; Jia, H.; Li, J.; Bu, L.; Qiao, B.; Zhao, X.; Jiang, Z. Conjugate Addition–Enantioselective Protonation of N-Aryl Glycines to α-Branched 2-Vinylazaarenes via Cooperative Photoredox and Asymmetric Catalysis. J. Am. Chem. Soc. 2018, 140, 6083–6087. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kong, M.; Qiao, B.; Lee, R.; Zhao, X.; Jiang, Z. Formal enantioconvergent substitution of alkyl halides via catalytic asymmetric photoredox radical coupling. Nat. Commun. 2018, 9, 2445. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Li, Y.; Gonçalves, T.P.; Zhan, Q.; Wang, G.; Zhao, X.; Qiao, B.; Huang, K.W.; Jiang, Z. All-Carbon Quaternary Stereocenters α to Azaarenes via Radical-Based Asymmetric Olefin Difunctionalization. J. Am. Chem. Soc. 2020, 142, 19451–19456. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wei, G.; Jiang, Z. Access to Isoxazolidines through Visible-Light-Induced Difunctionalization of Alkenes. ACS Catal. 2019, 9, 9599–9605. [Google Scholar] [CrossRef]
- Liu, X.; Yin, Y.; Jiang, Z. Photoredox-catalysed formal [3 + 2] cycloaddition of N-aryl α-amino acids with isoquinoline N-oxides. Chem. Commun. 2019, 55, 11527–11530. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Li, C.; Zhao, X.; Yin, Y.; Jiang, Z. Enantioselective reduction of azaarene-based ketones via visible light-driven photoredox asymmetric catalysis. Chem. Commun. 2019, 55, 7534–7537. [Google Scholar] [CrossRef]









 |  |  | |
|---|---|---|---|
| 1a | 1b | 1c | |
| λabs (nm) | 379 | 391 | 448 |
| ε[M−1cm−1] | 17,400 | 14,600 | 21,500 |
| E*red (V) | −1.36 | −1.00 | −1.07 |
| Eox (V) | 1.95 | 1.32 | 1.32 |
 increasing donor ability red shift absorption | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, V.; Singh, P.K.; Srivastava, A.; Sinha, S.; Singh, P.P. Recent Advances of Dicyanopyrazine (DPZ) in Photoredox Catalysis. Photochem 2021, 1, 237-246. https://doi.org/10.3390/photochem1020014
Srivastava V, Singh PK, Srivastava A, Sinha S, Singh PP. Recent Advances of Dicyanopyrazine (DPZ) in Photoredox Catalysis. Photochem. 2021; 1(2):237-246. https://doi.org/10.3390/photochem1020014
Chicago/Turabian StyleSrivastava, Vishal, Pravin K. Singh, Arjita Srivastava, Surabhi Sinha, and Praveen P. Singh. 2021. "Recent Advances of Dicyanopyrazine (DPZ) in Photoredox Catalysis" Photochem 1, no. 2: 237-246. https://doi.org/10.3390/photochem1020014