Soil-Transmitted Parasites and Non-Pathogenic Nematodes in Different Regions of Porto Alegre City, Brazil: A Comparison between Winter and Summer
Abstract
:1. Introduction
2. Results
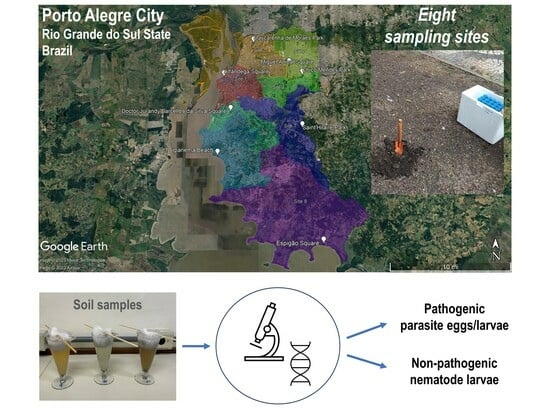
2.1. Characteristics of the Sampling Sites
2.2. Frequency of Parasite/Nematode-Positive Sites
2.3. Frequency of Microscopic Non-Pathogenic Nematodes and Pathogenic Parasites between Seasons
2.4. Molecular Analysis
3. Discussion
4. Materials and Methods
4.1. Legal Aspects
4.2. Study Area and Soil Sampling
4.3. Microscopy Analysis of Soil Samples
4.4. DNA Extraction and Molecular Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marcogliese, D.J. Parasites: Small players with crucial roles in the ecological theater. EcoHealth 2004, 1, 151–164. [Google Scholar] [CrossRef]
- Webster, J.P.; Kaushik, M.; Bristow, G.C.; McConkey, G.A. Toxoplasma gondii infection, from predation to schizophrenia: Can animal behaviour help us understand human behaviour? J. Exp. Biol. 2013, 216, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Manderson, L.; Aagaard-Hansen, J.; Allotey, P.; Gyapong, M.; Sommerfeld, J. Social research on neglected diseases of poverty: Continuing and emerging themes. PLoS Negl. Trop. Dis. 2009, 3, e332. [Google Scholar] [CrossRef] [PubMed]
- Ellwanger, J.H.; Fearnside, P.M.; Ziliotto, M.; Valverde-Villegas, J.M.; Veiga, A.B.G.; Vieira, G.F.; Bach, E.; Cardoso, J.C.; Müller, N.F.D.; Lopes, G.; et al. Synthesizing the connections between environmental disturbances and zoonotic spillover. An. Acad. Bras. Cienc. 2022, 94, e20211530. [Google Scholar] [CrossRef] [PubMed]
- Paula, F.M.; Costa-Cruz, J.M. Epidemiological aspects of strongyloidiasis in Brazil. Parasitology 2011, 138, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.S.; Mariano, A.P.M.; Silva, M.F. Combination of factors that increase the risk of contamination by geohelminths larvae in the south coast of Bahia, Brazil. BJD 2019, 5, 29254–29270. [Google Scholar] [CrossRef]
- Ziliotto, M.; Ellwanger, J.H.; Chies, J.A.B. Geo-helmintíases no Rio Grande do Sul: Uma análise a partir da perspectiva de Saúde Única. Bio. Diverso 2022, 2, 66–94. [Google Scholar]
- Ásbjörnsdóttir, K.H.; Means, A.R.; Werkman, M.; Walson, J.L. Prospects for elimination of soil-transmitted helminths. Curr. Opin. Infect. Dis. 2017, 30, 482–488. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Ziliotto, M.; Kulmann-Leal, B.; Chies, J.A.B. Iron deficiency and soil-transmitted helminth infection: Classic and neglected connections. Parasitol. Res. 2022, 121, 3381–3392. [Google Scholar] [CrossRef]
- Montresor, A.; Mwinzi, P.; Mupfasoni, D.; Garba, A. Reduction in DALYs lost due to soil-transmitted helminthiases and schistosomiasis from 2000 to 2019 is parallel to the increase in coverage of the global control programmes. PLoS Negl. Trop. Dis. 2022, 16, e0010575. [Google Scholar] [CrossRef]
- Shapiro, K.; Bahia-Oliveira, L.; Dixon, B.; Dumètre, A.; de Wit, L.A.; VanWormer, E.; Villena, I. Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food Waterborne Parasitol. 2019, 15, e00049. [Google Scholar] [CrossRef]
- Engroff, P.; Ely, L.S.; Guiselli, S.R.; Goularte, F.H.; Gomes, I.; Viegas, K.; De Carli, G.A. Soroepidemiologia de Toxoplasma gondii em idosos atendidos pela Estratégia Saúde da Família, Porto Alegre, Rio Grande do Sul, Brasil. Cien. Saude Colet. 2014, 19, 3385–3393. [Google Scholar] [CrossRef]
- Prüss-Ustün, A.; Wolf, J.; Bartram, J.; Clasen, T.; Cumming, O.; Freeman, M.C.; Gordon, B.; Hunter, P.R.; Medlicott, K.; Johnston, R. Burden of disease from inadequate water, sanitation and hygiene for selected adverse health outcomes: An updated analysis with a focus on low- and middle-income countries. Int. J. Hyg. Environ. Health 2019, 222, 765–777. [Google Scholar] [CrossRef]
- Prüss-Ustün, A.; Wolf, J.; Corvalán, C.; Neville, T.; Bos, R.; Neira, M. Diseases due to unhealthy environments: An updated estimate of the global burden of disease attributable to environmental determinants of health. J. Public Health 2017, 39, 464–475. [Google Scholar] [CrossRef]
- Campbell, S.J.; Biritwum, N.K.; Woods, G.; Velleman, Y.; Fleming, F.; Stothard, J.R. Tailoring Water, Sanitation, and Hygiene (WASH) Targets for Soil-Transmitted Helminthiasis and Schistosomiasis Control. Trends Parasitol. 2018, 24, 53–63. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Preventive Chemotherapy to Control Soil-Transmitted Helminth Infections in At-Risk Population Groups; World Health Organization: Geneva, Switzerland, 2017.
- Mara, D.; Lane, J.; Scott, B.; Trouba, D. Sanitation and health. PLoS Med. 2010, 7, e1000363. [Google Scholar] [CrossRef]
- Souza, C.M.N.; Costa, A.M.; Moraes, L.R.S.; de Freitas, M.C. Saneamento: Promoção Da Saúde, Qualidade De Vida E Sustentabilidade Ambiental; Editora FIORUZ: Rio de Janeiro, Brazil, 2015. [Google Scholar] [CrossRef]
- Ritchie, H.; Rosen, M. Our World in Data, Sanitation. Available online: https://ourworldindata.org/sanitation#:~:text=In%202020%2C%20just%20over%20half,have%20to%20practice%20open%20defecation (accessed on 28 May 2022).
- Instituto Trata Brasil. Ranking do Saneamento Básico 2017 (Sistema Nacional de Informações sobre Saneamento–SNIS 2015); Instituto Trata Brasil: São Paulo, Brazil, 2017. [Google Scholar]
- Instituto Trata Brasil. Ranking do Saneamento Básico 2022 (Sistema Nacional de Informações sobre Saneamento–SNIS 2020); Instituto Trata Brasil: São Paulo, Brazil, 2022. [Google Scholar]
- United Nations. Sustainable Development Goals, Goal 6: Ensure Access To Water and Sanitation for All. Available online: https://www.un.org/sustainabledevelopment/water-and-sanitation/ (accessed on 31 May 2023).
- Freeman, M.C.; Garn, J.V.; Sclar, G.D.; Boisson, S.; Medlicott, K.; Alexander, K.T.; Penakalapati, G.; Anderson, D.; Mahtani, A.G.; Grimes, J.E.T.; et al. The impact of sanitation on infectious disease and nutritional status: A systematic review and meta-analysis. Int. J. Hyg. Environ. Health 2017, 220, 928–949. [Google Scholar] [CrossRef]
- Ministério das Cidades Brasil; Organização Pan-Americana da Saúde. Programa de Modernização do Setor de Saneamento. In Política e Plano Municipal De Saneamento Ambiental: Experiências E Recomendações; OPAS: Brasília, Brazil, 2005. [Google Scholar]
- World Health Organization. Water, Sanitation and Hygiene (WASH). Available online: https://www.who.int/health-topics/water-sanitation-and-hygiene-wash#tab=tab_2 (accessed on 31 May 2023).
- Ellwanger, J.H.; Veiga, A.B.G.; Kaminski, V.L.; Valverde-Villegas, J.M.; Freitas, A.W.Q.; Chies, J.A.B. Control and prevention of infectious diseases from a One Health perspective. Genet. Mol. Biol. 2021, 44, e20200256. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.; Blackett, I.; Heymans, C. Poor-Inclusive Urban Sanitation: An Overview; International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2013. [Google Scholar]
- Azambuja, M.I.; Lewgoy, A.; Kolling, J.H.; Espíndola, I. Cidades, desigualdades e a dengue: Lições de uma grande epidemia de dengue numa microárea de Porto Alegre. In Estruturas e Dinâmicas Socioespaciais Urbanas no Rio Grande do Sul: Transformações em Tempos de Globalização (1991–2010); Heidrich, A.L., Soares, P.R.R., Tartaruga, I.G.P., Mammarella, R., Eds.; Organisation Observatório das Metrópoles–Núcleo Porto Alegre, Letra1 Editora: Porto Alegre, Brazil, 2016; pp. 261–284. [Google Scholar]
- Jagdale, G.B.; Davis, R.F.; Bertrand, P.; Gay, J.D.; Baird, R.E.; Padgett, G.B.; Brown, E.A.; Hendrix, F.F.; Balsdon, J.A. Guide for Interpreting Nematode Assay Results, Circular 834; The University of Georgia: Athens, GA, USA, 2013. [Google Scholar]
- Orgiazzi, A.; Bardgett, R.D.; Barrios, E.; Behan-Pelletier, V.; Briones, M.J.I.; Chotte, J.-L.; De Deyn, G.B.; Eggleton, P.; Fierer, N.; Fraser, T.; et al. (Eds.) Global Soil Biodiversity Atlas; European Commission, Publications Office of the European Union: Luxembourg, 2016.
- Carvalho, S.M.; Gonçalves, F.A.; Campos Filho, P.C.; Guimarães, E.M.; González Y Cáceres, A.P.; Souza, Y.B.; Vianna, L.C. Adaptação do método de Rugai e colaboradores para análise de parasitas do solo. Rev. Soc. Bras. Med. Trop. 2005, 38, 270–271. [Google Scholar] [CrossRef] [PubMed]
- Verschoor, B.C.; de Goede, R.G.M.; de Hoop, J.W.; de Vries, F.W. Seasonal dynamics and vertical distribution of plant-feeding nematode communities in grasslands. Pedobiologia 2001, 45, 213–233. [Google Scholar] [CrossRef]
- Cociancic, P.; Torrusio, S.E.; Garraza, M.; Zonta, M.L.; Navone, G.T. Intestinal parasites in child and youth populations of Argentina: Environmental factors determining geographic distribution. Rev. Argent. Microbiol. 2021, 53, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Elias, J.; Ferraz, C. Rio Grande do Sul Tem Terceiro Ano De Seca E “Isso Não É Normal’, Diz Professor. Available online: https://www.cnnbrasil.com.br/nacional/rio-grande-do-sul-tem-terceiro-ano-de-seca-e-isso-nao-e-normal-diz-professor/ (accessed on 29 May 2023).
- Sias, E. Pior seca no mundo hoje é da Argentina, Uruguai e Rio Grande do Sul. Available online: https://metsul.com/pior-seca-no-mundo-hoje-e-da-argentina-uruguai-e-rio-grande-do-sul/ (accessed on 29 May 2023).
- Ma, Q.; Yu, H.; Liu, X.; Xu, Z.; Zhou, G.; Shi, Y. Climatic warming shifts the soil nematode community in a desert steppe. Clim. Change 2018, 150, 243–258. [Google Scholar] [CrossRef]
- Singh, J.; Schädler, M.; Demetrio, W.; Brown, G.G.; Eisenhauer, N. Climate change effects on earthworms—A review. Soil. Org. 2019, 91, 114–138. [Google Scholar] [CrossRef] [PubMed]
- Siebert, J.; Ciobanu, M.; Schädler, M.; Eisenhauer, N. Climate change and land use induce functional shifts in soil nematode communities. Oecologia 2020, 192, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Tibbett, M.; Fraser, T.D.; Duddigan, S. Identifying potential threats to soil biodiversity. PeerJ 2020, 8, e9271. [Google Scholar] [CrossRef]
- Short, E.E.; Caminade, C.; Thomas, B.N. Climate change contribution to the emergence or re-emergence of parasitic diseases. Infect. Dis. 2017, 10, 1178633617732296. [Google Scholar] [CrossRef]
- Vargas, M.M.; De Bastiani, M.; Ferreira, J.R.D.; Calil, L.N.; Spalding, S.M. Frequência de estruturas parasitárias em praças e parques públicos da cidade de Porto Alegre-RS. Rev. Patolog. Trop. 2013, 42, 434–442. [Google Scholar] [CrossRef]
- Mentz, M.B.; Rott, M.B.; Jacobsen, S.I.V.; Baldo, G.; Rodrigues-Júnior, V. Frequência de ovos de Toxocara spp. Em três parques públicos da cidade de Porto Alegre, Rio Grande do Sul, Brasil. Rev. Patolog. Trop. 2004, 33, 105–112. [Google Scholar] [CrossRef]
- Fernandes de Oliveira, P.R.; de Melo, R.P.B.; Sierra, T.A.O.; da Silva, R.A.; da Silva de Oliveira, J.E.; de Almeida, B.G.; Mota, R.A. Investigation of soil contaminated with Toxoplasma gondii oocyst in urban public environment, in Brazil. Comp. Immunol. Microbiol. Infect. Dis. 2021, 79, 101715. [Google Scholar] [CrossRef]
- Fernandes de Oliveira, P.R.; de Melo, R.P.B.; de Oliveira, U.D.R.; Magalhães, F.J.R.; Junior, R.J.F.; Andrade, M.R.; Mota, R.A. Detection of Toxoplasma gondii oocysts in soil and risk mapping in an island environment in the Northeast of Brazil. Transbound Emerg. Dis. 2022, 69, 3457–3467. [Google Scholar] [CrossRef]
- Gnani-Charitha, V.; Rayulu, V.; Kondaiah, P.; Srilatha, C. Comparative evaluation of flotation techniques for the detection of soil borne parasites. J. Parasit. Dis. 2013, 37, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Lescano, S.A.Z.; Chieffi, P.P.; Peres, B.A.; de Mello, E.O.; Velarde, C.N.; Salinas, A.A.; Rojas, C.E. Soil contamination and human infection by Toxocara sp. in the urban area of Lima, Peru. Mem. Inst. Oswaldo 1988, 93, 733–734. [Google Scholar] [CrossRef] [PubMed]
- Villafañe-Ferrer, L.M.; Pinilla-Pérez, M. Intestinal parasites in children and soil from Turbaco, Colombia and associated risk factors. Rev. Salud Publica 2016, 18, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Rivero, M.R.; De Angelo, C.; Nuñez, P.; Salas, M.; Motta, C.E.; Chiaretta, A.; Salomón, O.D.; Liang, S. Environmental and socio-demographic individual, family and neighborhood factors associated with children intestinal parasitoses at Iguazú, in the subtropical northern border of Argentina. PLoS Negl. Trop. Dis. 2017, 11, e0006098. [Google Scholar] [CrossRef] [PubMed]
- Falcone, A.C.; Zonta, M.L.; Unzaga, J.M.; Navone, G.T. Agricultural practices and intestinal parasites: A study of socio-environmental risk factors associated with leafy vegetable production in La Plata horticultural area, Argentina. Parasite Epidemiol. Control 2023, 23, e00327. [Google Scholar] [CrossRef] [PubMed]
- Ellwanger, J.H.; Bach, E.; Müller, N.F.D.; Cardoso, J.C.; Seger, G.D.S.; Chies, J.A.B. Diversity of mosquitoes from Porto Alegre region, Rio Grande do Sul, Brazil: Ecological and public health perspectives. J. Insect. Conserv. 2022, 26, 873–891. [Google Scholar] [CrossRef]
- Prefeitura Municipal de Porto Alegre; Secretaria do Planejamento Municipal. Plano Diretor de Desenvolvimento Urbano Ambiental (PDDUA). Available online: https://www2.portoalegre.rs.gov.br/spm/default.php?reg=2&p_secao=205 (accessed on 31 January 2023).
- Ziliotto, M.; Ellwanger, J.H.; Chies, J.A.B. Soil-transmitted helminths detected from environmental samples in a campus of southern Brazil. Sci. One Health 2022, 1, 100016. [Google Scholar] [CrossRef]
- Ramos, I.C.d.N.; Lima, T.A.R.F.; Ramos, R.A.d.N.; Carvalho, G.A.d.; Alves, L.C. Contamination by Eggs of Nematodes (Nematoda) of Public Health Concern in Tropical Beaches. Parasitologia 2022, 2, 95–100. [Google Scholar] [CrossRef]
- Holmström, O.; Linder, N.; Ngasala, B.; Mårtensson, A.; Linder, E.; Lundin, M.; Moilanen, H.; Suutala, A.; Diwan, V.; Lundin, J. Point-of-care mobile digital microscopy and deep learning for the detection of soil-transmitted helminths and Schistosoma haematobium. Glob. Health Action 2017, 10, 1337325. [Google Scholar] [CrossRef]
- Rugai, E.; Mattos, T.; Brisola, A.P. Nova técnica para isolar larvas de nematóides das fezes; modificação do método de Baermann. Rev. Inst. Adolfo Lutz 1954, 14, 5–8. [Google Scholar]
- Neves, D.P. (Ed.) Parasitologia Dinâmica, 3rd ed.; Atheneu: São Paulo, Brazil, 2009. [Google Scholar]
- De Carli, G.A. (Ed.) Parasitologia Clínica: Seleção De Métodos E Técnicas De Laboratório Para O Diagnóstico Das Parasitoses Humanas, 2nd ed.; Atheneu: São Paulo, Brazil, 2011. [Google Scholar]
- Mariano, M.L.M.; Mariano, A.P.M.; Silva, M.M. (Eds.) Manual de Parasitologia Humana, 3rd ed.; Editus: Ilhéus, Brazil, 2016. [Google Scholar]
- CDC, Centers for Disease Control and Prevention; Global Health, Division of Parasitic Diseases and Malaria. DPDx—Laboratory Identification of Parasites of Public Health Concern. Available online: https://www.cdc.gov/dpdx/index.html (accessed on 5 December 2022).
- Ministério da Saúde; Universidade Estadual de Londrina. Protocolos Para Investigação de Toxoplasma Gondii em Amostras Ambientais e Alimentares; Ministério da Saúde, Universidade Estadual de Londrina: Paraná, Brazil, 2020.
- Homan, W.L.; Vercammen, M.; De Braekeleer, J.; Verschueren, H. Identification of a 200- to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int. J. Parasitol. 2000, 30, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.H. WINPEPI updated: Computer programs for epidemiologists, and their teaching potential. Epidemiol. Perspect. Innov. 2011, 8, 1. [Google Scholar] [CrossRef] [PubMed]

| Sampling Sites | Popular Name 1 | Latitude | Longitude | Temperature (Average), Winter | Temperature (Average), Summer | Weather Condition, Winter | Weather Condition, Summer | Soil Characteristic |
|---|---|---|---|---|---|---|---|---|
| Site 1 | Alfândega Square | −30.028917 | −51.230997 | 13 °C | 26 °C | Cloudy | Cloudy | Organic; Sandy |
| Site 2 | Mascarenhas de Moraes Park | −29.981681 | −51.186776 | 20 °C | 28 °C | Cloudy | Sunny | Organic; Sandy; Clayey |
| Site 3 | Miguel Anibal Square | −30.012382 | −51.132493 | 13 °C | 25 °C | Cloudy | Cloudy | Organic; Sandy; Clayey |
| Site 4 | Chico Mendes Park | −30.026889 | −51.112667 | 21 °C | 22 °C | Cloudy | Cloudy | Organic; Sandy; Clayey |
| Site 5 | Doctor Jurandy Barcellos da Silva Square | −30.076251 | −51.219721 | 20 °C | 28 °C | Cloudy | Sunny | Organic; Sandy; Clayey |
| Site 6 | Ipanema Beach 2 | −30.133273 | −51.237503 | 18 °C | 28 °C | Cloudy | Sunny | Organic; Sandy |
| Site 7 | Saint’Hilaire Park | −30.097943 | −51.113585 | 15 °C | 27 °C | Cloudy | Sunny | Organic; Clayey |
| Site 8 | Espigão Square | −30.241057 | −51.086015 | 20 °C | 25 °C | Sunny | Sunny | Organic; Sandy; Clayey |
| Sampling Sites | Local Vegetation | Human Dwellings/Buildings | Noise Pollution | Environmental Sanitation | Domestic Sewage Disposal | Artificial Mosquito Larvae Breeding Grounds | Domestic Solid Waste | Industrial/Biological Sewage Disposal | Expected Human Circulation | Domestic and Farm Animals 1 | Synanthropic Animals 1 | Human Feces |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site 1 | Ornamental | Regular | Present | Proper | Absent | Present | Present | Absent | Intense | Present, dogs | Present, pigeons and rodents | Present |
| Site 2 | Ornamental | Regular | Absent/light sounds | Insufficient | Present | Present | Present | Absent | Frequent | Present, dogs | Present, pigeons | Present |
| Site 3 | Ornamental | Regular | Present | Proper | Absent | Present | Present | Absent | Infrequent | Present, dogs | Present, pigeons | Absent |
| Site 4 | Ornamental | Regular | Absent/light sounds | Proper | Absent | Present | Present | Absent | Frequent | Present, dogs | Present, pigeons | Absent |
| Site 5 | Ornamental | Regular | Present | Proper | Absent | Present | Present | Absent | Frequent | Present, dogs and cats | Present, pigeons | Present |
| Site 6 | Naturally absent | Regular | Present | Insufficient | Present | Present | Present | Absent | Frequent | Present, dogs | Present, pigeons | Absent |
| Site 7 | Natural grasslands/shrubs and preserved arboreal forest | Irregular | Absent/lights sounds | Insufficient | Present | Absent | Present | Absent | Infrequent | Present, dogs and horses | Absent | Absent |
| Site 8 | Ornamental and degraded arboreal forest | Regular | Present | Proper | Absent | Present | Present | Absent | Infrequent | Present, dogs | Absent | Absent |
| Observed Parasite Larvae and Eggs | Sampling Sites | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Site 7 | Site 8 | |||||||||
| Winter (n = 10) | Summer (n = 10) | Winter (n = 10) | Summer (n = 10) | Winter (n = 10) | Summer (n = 10) | Winter (n = 10) | Summer (n = 10) | Winter (n = 10) | Summer (n = 10) | Winter (n = 10) | Summer (n = 10) | Winter (n = 10) | Summer (n = 10) | Winter (n = 10) | Summer (n = 10) | |
| Non-pathogenic nematode larvae 1 | +(10) | +(9) | +(9) | +(8) | +(10) | +(9) | +(10) | +(10) | +(9) | +(9) | +(9) | +(3) | +(9) | +(8) | +(9) | +(10) |
| Hookworm (filariform) larvae | +(1) | +(1) | ||||||||||||||
| Hookworm (rhabditiform) larvae | +(1) | +(1) | +(1) | +(1) | +(2) | +(2) | +(2) | +(2) | +(1) | +(1) | +(3) | +(1) | ||||
| Strongyloides spp. (filariform) larvae | +(1) | |||||||||||||||
| Strongyloides spp. (rhabditiform) larvae | +(1) | +(1) | +(1) | +(1) | ||||||||||||
| Hookworm eggs 2 | +(1) | +(1) | +(1) | +(2) | +(2) | +(1) | +(1) | +(1) | +(2) | +(1) | +(3) | +(1) | ||||
| Ascaris spp. eggs | +(1) | +(1) | +(1) | +(1) | +(1) | +(2) | +(2) | +(2) | +(1) | +(3) | +(1) | |||||
| Trichuris spp. eggs | +(2) | |||||||||||||||
| Observed Parasite Larvae and Eggs | Winter + Summer (n = 160) | Winter (n = 80) | Summer (n = 80) | Fisher’s p-Value (Winter versus Summer) |
|---|---|---|---|---|
| Microscopic nematode larvae 1 | 141 (88.13%) | 75 (93.75%) | 66 (82.50%) | 0.048 |
| Hookworm (filariform) larvae | 2 (1.25%) | 1 (1.25%) | 1 (1.25%) | 1.000 |
| Hookworm (rhabditiform) larvae | 18 (11.25%) | 12 (15.00%) | 6 (7.50%) | 0.210 |
| Strongyloides spp. (filariform) larvae | 1 (0.63%) | 1 (1.25%) | 0 (0%) | 1.000 |
| Strongyloides spp. (rhabditiform) larvae | 4 (2.5%) | 2 (2.50%) | 2 (2.50%) | 1.000 |
| Hookworm eggs 2 | 17 (10.63%) | 11 (13.75%) | 6 (7.50%) | 0.305 |
| Ascaris spp. eggs | 16 (10.00%) | 9 (11.25%) | 7 (8.75%) | 0.793 |
| Trichuris spp. eggs | 2 (1.25%) | 2 (2.50%) | 0 (0%) | 0.497 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziliotto, M.; Ellwanger, J.H.; Chies, J.A.B. Soil-Transmitted Parasites and Non-Pathogenic Nematodes in Different Regions of Porto Alegre City, Brazil: A Comparison between Winter and Summer. Parasitologia 2024, 4, 1-14. https://doi.org/10.3390/parasitologia4010001
Ziliotto M, Ellwanger JH, Chies JAB. Soil-Transmitted Parasites and Non-Pathogenic Nematodes in Different Regions of Porto Alegre City, Brazil: A Comparison between Winter and Summer. Parasitologia. 2024; 4(1):1-14. https://doi.org/10.3390/parasitologia4010001
Chicago/Turabian StyleZiliotto, Marina, Joel Henrique Ellwanger, and José Artur Bogo Chies. 2024. "Soil-Transmitted Parasites and Non-Pathogenic Nematodes in Different Regions of Porto Alegre City, Brazil: A Comparison between Winter and Summer" Parasitologia 4, no. 1: 1-14. https://doi.org/10.3390/parasitologia4010001