Mechanical Characterization of Anhydrous Microporous Aluminophosphate Materials: Tridimensional Incompressibility, Ductility, Isotropy and Negative Linear Compressibility
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
3.1. Crystal Structures
3.2. Stiffness Tensors and Mechanical Stability
3.3. Mechanical Properties
3.4. Mechanical Properties as a Function of the Orientation of the Applied Strain
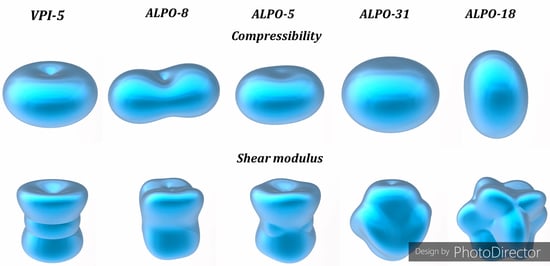
3.5. Compressibility Functions
3.6. Negative Linear Compressibility (NLC) in ALPO-18
3.6.1. Isotropic Negative Linear Compressibility (INLC)
3.6.2. Anisotropic Negative Volumetric Compressibility (ANVC) Effect
3.6.3. Effect of Dispersion Interactions in the NLC Effect of ALPO-18
3.7. Effect of Hydration in the Mechanical Properties of ALPO-18
3.8. Effect of Aging in the Elastic Properties of VPI-5
3.9. Effect of Pressure Polymorphism
3.9.1. VPI-5
3.9.2. ALPO-5
3.10. Comparison with Experimental Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wright, P.A. Microporous Framework Solids; RSC Publishing: Cambridge, UK, 2008. [Google Scholar]
- Davis, M.E.; Garces, J.; Saldarriaga, C.; Crowder, C. A molecular sieve with eighteen-membered rings. Nature 1988, 331, 698–699. [Google Scholar] [CrossRef]
- McCusker, L.B.; Baerlocher, C.; Jahn, E.; Bülow, M. The Triple Helix inside the Large-Pore Aluminophosphate Molecular Sieve VPI-5. Zeolites 1991, 11, 308–313. [Google Scholar] [CrossRef]
- Poojary, D.M.; Perez, D.M.; Clearfield, J.O. Crystal Structures of Dehydrated VPI-5 and H1 Aluminum Phosphates from X-ray Powder Data. J. Phys. Chem. C 1992, 96, 7709–7714. [Google Scholar] [CrossRef]
- De Oñate Martinez, J.; McCusker, L.B.; Baerlocher, C. Characterization and Structural Analysis of Differently Pre-pared Samples of Dehydrated VPI-5. Microporous Mesoporous Mater. 2000, 34, 99–113. [Google Scholar] [CrossRef]
- Dessau, R.M.; Schlenker, J.L.; Higgins, J.B. Structure Determination and Rietveld Refinement of Aluminophosphate Molecular Sieve AlPO4-8. Zeolites 1990, 10, 522–524. [Google Scholar] [CrossRef]
- Richardson, J.W.; Vogt, E.T.C. Structure determination and rietveld refinement of aluminophosphate molecular sieve AIPO4-8. Zeolites 1992, 12, 13–19. [Google Scholar] [CrossRef]
- Mora, A.J.; Fitch, A.N.; Cole, M.; Goyal, R.; Jones, R.H.; Jobic, H.C.; Carr, S.W. The Structure of the Calcined Aluminophosphate AlPO4-5 Determined by High Resolution X-Ray and Neutron Powder Diffraction. J. Mater. Chem. 1996, 6, 1831–1835. [Google Scholar] [CrossRef]
- Klap, G.J.; van Koningsveld, H.; Graafsma, H.; Schreurs, A.M.M. Absolute Configuration and Domain Structure of AlPO4-5 Studied by Single Crystal X-ray Diffraction. Microporous Mesoporous Mater. 2000, 38, 403–412. [Google Scholar] [CrossRef]
- Simmen, A.; McCusker, L.B.; Baerlocher, C.W.; Meier, W. The Structure Determination and Rietveld Refinement of the Aluminophosphate AlPO4-18. Zeolites 1991, 11, 654–661. [Google Scholar] [CrossRef]
- Poulet, G.; Tuelm, A.; Sautet, P.A. Combined Experimental and Theoretical Evaluation of the Structure of Hydrated Microporous Aluminophosphate AlPO4-18. J. Phys. Chem. B 2005, 109, 22939–22946. [Google Scholar] [CrossRef]
- Bennett, J.M.; Kirchner, R.M. The Structure of Calcined AlPO4-31: A New Framework Topology Containing One-Dimensional 12-Ring Pores. Zeolites 1992, 12, 338–342. [Google Scholar] [CrossRef]
- Endregard, M.; Nicholson, D.G.; Stocker, M.; Beagle, B. Cobalticenium Ions Adsorbed on Large-pore Aluminophosphate VPI-5 Studied by X-Ray Absorption, 13CSolid-state NMR and FTlR Spectroscopy. J. Mater. Chem. 1995, 5, 485–491. [Google Scholar] [CrossRef]
- Endregard, M.; Nicholson, D.G.; Stocker, M.; Lamble, G.J. Adsorption and Thermal Decomposition of Cobalticenium Ions on AlPO4-5 Studied by X-Ray Absorption Spectroscopy, 13C Solid-State NMR and FTlR. J. Mater. Chem. 1995, 5, 785–791. [Google Scholar] [CrossRef]
- Parton, R.F.; Thibault-Starzyk, F.; Reynders, R.A.; Grobet, P.J.; Jacobs, P.A.; Bezoukhanova, C.P.; Sun, W.; Wu, Y. Stacked Phthalocyanines in VPI-5 Pores as Evidenced by CPDOR 1H27Al NMR. Mol. Catal. A 1995, 97, 183–186. [Google Scholar] [CrossRef]
- Kärger, J.; Keller, W.; Pfeifer, H.; Ernst, S.; Weitkamp, J. Unexpectedly Low Translational Mobility of Methane and Tetrafluoromethane in the Large-Pore Molecular Sieve VPI-5. Microporous Mater. 1995, 3, 401–408. [Google Scholar] [CrossRef]
- Jin, Y.M.; Chon, H. A Novel Method for Encapsulation of Dyes into AlPO4-8 Molecular Sieve. Chem. Commun. 1996, 1996, 135–136. [Google Scholar] [CrossRef]
- Ganschow, M.; Schulz-Ekloff, G.; Wark, M.; Wendschuh-Josties, M.; Wöhrle, D. Microwave-Assisted Preparation of Uniform Pure and Dye-Loaded AlPO4-5 Crystals with Different Morphologies for Use as Microlaser Systems. J. Mater. Chem. 2001, 11, 1823–1827. [Google Scholar] [CrossRef]
- Gonzalez-Platas, J.; Breton, J.; Girardet, C. Physisorption in a Molecular Helicoidal Cavity: Application to AlPO4-5. Langmuir 1995, 11, 197–203. [Google Scholar] [CrossRef]
- Hartmann, M.; Kevan, L. Generation of Ion-Exchange Capacity by Silicon Incorporation into the Aluminophosphate VPI-5/AlPO4-5 Molecular Sieve System. J. Chem. Soc. Faraday Trans. 1996, 92, 3661–3667. [Google Scholar] [CrossRef]
- Garcia-Carmona, J.; Fanovich, M.A.; Llibre, J.; Rodriguez-Clemente, R.; Domingo, C. Processing of Microporous VPI-5 Molecular Sieve by Using Supercritical CO2: Stability and Adsorption Properties. Microporous Mesoporous Mater. 2000, 54, 127–137. [Google Scholar] [CrossRef]
- Van Heyden, H.; Mintova, S.; Bein, T. AlPO4-18 Nanocrystals Synthesized Under Microwave Irradiation. J. Mater. Chem. 2006, 16, 514–518. [Google Scholar] [CrossRef]
- Shutilov, A.; Grenev, I.V.; Kikhtyanin, O.V.; Gavrilov, V.Y. Adsorption of Molecular Hydrogen on Aluminophosphate Zeolites at 77 K. Kinet. Catal. 2012, 53, 137–144. [Google Scholar] [CrossRef]
- Weiß, O.; Loerke, J.; Wüstefeld, U.; Marlow, F.; Schüth, F.J. Host-Guest Interactions and Laser Activity in AlPO4-5/Laser Dye Composites. J. Solid State Chem. 2002, 67, 302–309. [Google Scholar] [CrossRef]
- Yao, M.; Wang, T.; Yao, Z.; Duan, D.; Chen, S.; Liu, Z.; Liu, R.; Lu, S.; Yuan, Y.; Zou, B.; et al. Pressure-Driven Topological Transformations of Iodine Confined in One-Dimensional Channels. J. Phys. Chem. C 2013, 117, 25052–25058. [Google Scholar] [CrossRef]
- Guo, J.; Wang, C.; Xu, J.; Deng, J.; Yan, R.P.; Sharma, R.; Xu, R. Encapsulation of Bulky Solvent Molecules into the Channels of Aluminophosphate Molecular Sieve and its Negative Influence on the Thermal Stability of Open-Framework. Inorg. Chem. Commun. 2018, 91, 67–71. [Google Scholar] [CrossRef]
- Carreon, M.L.; Li, S.; Carreon, M.A. AlPO4-18 Membranes for CO2/CH4 Separation. Chem. Commun. 2012, 48, 2310–2312. [Google Scholar] [CrossRef]
- Wu, T.; Lucero, J.; Zong, Z.; Elsaidi, S.K.; Thallapally, P.K.; Carreon, M.A. Microporous Crystalline Membranes for Kr/Xe Separation: Comparison Between ALPO-18, SAPO-34, and ZIF-8. ACS Appl. Nano Mater. 2018, 1, 463–470. [Google Scholar] [CrossRef]
- Wang, B.; Gao, F.; Zhang, F.; Xing, W.; Zhou, R. Highly Permeable and Oriented AlPO4-18 Membranes Prepared Using Directly Synthesized Nanosheets for CO2/CH4 Separation. J. Mater. Chem. A 2019, 7, 13164–13172. [Google Scholar] [CrossRef]
- Tang, Z.K.; Sun, H.D.; Wang, J.; Li, J.C. Silver oxalate: Mechanical properties and extreme negative mechanical phenomena. Appl. Phys. Lett. 1998, 73, 2287–2289. [Google Scholar] [CrossRef]
- Launois, P.; Moret, R.; Le Bolloc’h, D.; Albouya, P.A.; Tang, Z.K.; Li, G. Carbon Nanotubes Synthesised in Channels of AlPO4-5 Single Crystals: First X-ray Scattering Investigations. Solid State Commun. 2000, 116, 99–103. [Google Scholar] [CrossRef]
- Li, Z.M.; Tang, Z.K.; Liu, H.J.; Wang, N.; Chan, C.T.; Saito, R.; Okada, S.; Li, G.D.; Chen, J.S.; Nagasawa, N.; et al. Polarized Absorption Spectra of Single-Walled 4 Å Carbon Nanotubes Aligned in Channels of an AlPO4-5 Single Crystal. Phys. Rev. Lett. 2001, 87, 127401. [Google Scholar] [CrossRef] [PubMed]
- Li, G.D.; Tang, Z.K.; Wang, N.; Chen, J.S. Structural Study of the 0.4-nm Single-Walled Carbon Nanotubes Aligned in Channels of AlPO4-5 crystal. Carbon 2002, 40, 917–921. [Google Scholar]
- Zhai, J.P.; Tang, Z.K.; Li, Z.M.; Li, I.L.; Jiang, F.Y.; Shen, P.; Hu, X. Carbonization Mechanism of Tetrapropylammonium-hydroxide in Channels of AlPO4-5 Single Crystals. Chem. Mater. 2006, 18, 1505–1511. [Google Scholar] [CrossRef]
- Zhai, J.P.; Li, Z.M.; Liu, H.J.; Li, I.L.; Sheng, P.; Hu, H.J.; Tang, Z.K. Catalytic Effect of Metal Cations on the Formation of Carbon Nanotubes inside the Channels of AlPO4-5 crystal. Carbon 2006, 44, 1151–1157. [Google Scholar] [CrossRef]
- Yang, W.; Sun, W.; Zhao, S.; Yin, X. Single-Walled Carbon Nanotubes Prepared in Small AlPO4-5 and CoAPO-5 Molecular Sieves by Low-Temperature Hydrocracking. Microporous Mesoporous Mater. 2016, 219, 87–92. [Google Scholar] [CrossRef]
- Concepcion, P.; Lopez Nieto, J.M. Novel Synthesis of Vanadium Cobalt Aluminophosphate Molecular Sieve of AEI Structure (VCoAPO-18) and its Catalytic Behavior for Ethane Oxidation. Catal. Commun. 2001, 2, 363–367. [Google Scholar] [CrossRef]
- Concepcion, P.; Blasco, T.; Lopez Nieto, J.M.; Vidal-Moya, A.; Martinez-Arias, A. Preparation, Characterization and Reactivity of V- and/or Co-Containing AlPO4-18 Materials (VCoAPO-18) in the Oxidative Dehydrogenation of Ethane. Microporous Mesoporous Mater. 2004, 67, 215–227. [Google Scholar] [CrossRef]
- Dai, W.; Li, N.; Guan, N.; Hunger, M. Unexpected Methanol-to-Olefin Conversion Activity of Low-Silica Aluminophosphate Molecular Sieves. Catal. Commun. 2011, 16, 124–127. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, B.; Liu, X.; Li, J. Cyclohexane Oxidation over AFI Molecular Sieves: Effects of Cr, Co Incorporation and Crystal Size. Catal. Sci. Technol. 2015, 5, 3394–3402. [Google Scholar] [CrossRef]
- Preeth, M.E.; Umasankari, A.; Rekha, C.H.; Palanichamy, P.; Sivakumar, T.; Pandurangan, A. Selective Oxidation of Cyclohexane to KA Oil Over Ce-AlPO-18 Molecular Sieves. Int. J. Eng. Technol. 2018, 7, 352–354. [Google Scholar] [CrossRef]
- Chen, J.; Thomas, J.M.; Wright, P.A. Silicoaluminophosphate Number Eighteen (SAPO-18): A New Microporous Solid Acid Catalyst. Catal. Lett. 1994, 28, 241–248. [Google Scholar] [CrossRef]
- Thomas, J.M.; Greaves, G.N.; Sanka, G.; Wright, P.A.; Chen, J.; Dent, A.J.; Marchese, L. On the Nature of the Active Site in a CoAPO-18 Solid Acid Catalyst. Angew. Chem. Int. Ed. 1994, 33, 1871–1873. [Google Scholar] [CrossRef]
- Zubowa, H.L.; Richter, M.; Roost, U.; Parlitz, B.; Fricke, R. Synthesis and Catalytic Properties of Substituted AlPO4-31 Molecular Sieves. Catal. Lett. 1993, 19, 67–79. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, D.; Li, F.; Gao, F.; Feng, C.; Wen, S.; Ruan, S. Humidity Sensor Based on AlPO4-5 Zeolite with High Responsivity and its Sensing Mechanism. Sens. Actuators B 2015, 212, 242–247. [Google Scholar] [CrossRef]
- Ristic, A.; Logar, N.Z.; Henninger, S.K.; Kaucic, V. The Performance of Small-Pore Microporous Aluminophosphates in Low-Temperature Solar Energy Storage: The Structure–Property Relationship. Adv. Funct. Mater. 2012, 22, 1952–1957. [Google Scholar] [CrossRef]
- Henninger, S.K.; Schmidt, F.P.; Henning, H.M. Water Adsorption Characteristics of Novel Materials for Heat Transformation Applications. Applied Thermal Eng. 2010, 30, 1692–1702. [Google Scholar] [CrossRef]
- Henninger, S.K.; Schmidt, F.P.; Henning, H.M. Characterisation and Improvement of Sorption Materials with Molecular Modeling for the Use in Heat Transformation Applications. Adsorption 2011, 17, 833–843. [Google Scholar] [CrossRef]
- Henninger, S.K.; Jeremias, F.; Kummer, H.; Schossig, P.; Henning, H.M. Novel Sorption Materials for Solar Heating and Cooling. Energy Procedia 2012, 30, 279–288. [Google Scholar] [CrossRef]
- Khosrovani, N.; Sleight, A.W. Flexibility of Network Structures. J. Solid State Chem. 1996, 121, 2–11. [Google Scholar] [CrossRef]
- Grima, J.N.; Jackson, R.; Alderson, A.; Evans, K.E. Do Zeolites Have Negative Poisson’s Ratios? Adv. Mater. 2000, 12, 1912–1918. [Google Scholar] [CrossRef]
- Grima, J.N.; Gatt, R.; Zammit, V.; Williams, J.J.; Evans, K.E.; Alderson, A.; Walton, R.I. Natrolite: A Zeolite with Negative Poisson’s Ratios. J. Appl. Phys. 2007, 101, 086102. [Google Scholar] [CrossRef]
- Sanchez-Valle, C.; Lethbridge, Z.A.D.; Sinogeikin, S.V.; Williams, J.J.; Walton, R.I.; Evans, K.E.; Bass, J.D. Negative Poisson’s Ratios in Siliceous Zeolite MFI-Silicalite. J. Chem. Phys. 2008, 128, 184503. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Valle, C.; Sinogeikin, V.; Lethbridge, Z.A.D.; Walton, R.I.; Smith, C.W.; Evans, K.E.; Bass, J.D. Brillouin Scattering Study on the Single Crystal of Natrolite and Analcime Zeolites. J. Appl. Phys. 2005, 98, 53508. [Google Scholar] [CrossRef]
- Eroshenko, V.; Regis, R.C.; Soulard, M.; Patarin, J. Energetics: A New Field of Applications for Hydrophobic Zeolites. J. Am. Chem. Soc. 2001, 123, 8129–8130. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Hriljac, J.A.; Vogt, T.; Parise, J.B.; Artioli, G. Phase Transition of Zeolite Rho at High-Pressure. J. Am. Chem. Soc. 2001, 123, 12732–12733. [Google Scholar] [CrossRef]
- Lee, Y.; Vogt, T.; Hriljac, J.A.; Parise, J.B.; Artioli, G. Pressure-Induced Volume Expansion of Zeolites in the Natrolite Family. J. Am. Chem. Soc. 2002, 124, 5466–5475. [Google Scholar] [CrossRef]
- Gatta, G.D.; Lee, Y. Zeolites at high pressure: A review. Mineral. Mag. 2014, 78, 267–291. [Google Scholar] [CrossRef]
- Arletti, R.; Ferro, O.; Quartieri, S.; Sani, A.; Tabacchi, G.; Vezzalini, G. Structural Deformation Mechanisms of Zeolites under Pressure. Am. Mineral. 2003, 88, 1416–1422. [Google Scholar] [CrossRef]
- Astala, R.; Auerbach, S.M.; Monson, P.A. Density Functional Theory Study of Silica Zeolite Structures: Stabilities and Mechanical Properties of SOD, LTA, CHA, MOR, and MFI. J. Phys. Chem. B 2004, 108, 9208–9215. [Google Scholar] [CrossRef]
- Astala, R.; Auerbach, S.M.; Monson, P.A. Normal mode approach for predicting the mechanical properties of solids from first principles: Application to compressibility and thermal expansion of zeolites. Phys. Rev. B 2005, 71, 014112. [Google Scholar] [CrossRef]
- Sartbaeva, A.; Wells, S.A.; Tracy, M.M.J.; Thorpe, M.F. The flexibility window in zeolites. Nat. Mater. 2006, 5, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, R.E.; Wells, S.A.; Leung, K.M.; Edwards, P.P.; Sartbaeva, A. Intrinsic flexibility of porous materials; theory, modelling and the flexibility window of the EMT zeolite framework. Acta Crystallogr. B 2015, 71, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Coudert, F.X. Systematic investigation of the mechanical properties of pure silicazeolites: Stiffness, anisotropy, and negative linear compressibility. Phys. Chem. Chem. Phys. 2013, 15, 16012–16018. [Google Scholar] [CrossRef]
- Evans, J.D.; Coudert, F.X. Predicting the Mechanical Properties of Zeolite Frameworks by Machine Learning. Chem. Mater. 2017, 29, 7833–7839. [Google Scholar] [CrossRef]
- Gaillac, R.; Chibani, S.; Coudert, F.X. Speeding up discovery of auxetic zeolite frameworks by machine learning. Chem. Mater. 2020, 32, 2653–2663. [Google Scholar] [CrossRef]
- Santoro, M.; Veremeienko, V.; Polisi, M.; Fantini, R.; Alabarse, F.; Arletti, R.; Quatieri, S.; Svitlyk, V.; Van der Lee, A.; Rouquette, J.; et al. Insertion and Confinement of H2O in Hydrophobic Siliceous Zeolites at High Pressure. J. Phys. Chem. C 2019, 123, 17432–17439. [Google Scholar] [CrossRef]
- Bahr, D.F.; Reid, J.A.; Mook, W.M.; Bauer, C.A.; Stumpf, R.; Skulan, A.J.; Moody, N.R.; Simmons, B.A.; Shindel, M.M.; Allendorf, M.D. Mechanical properties of cubic zinc carboxylate IRMOF-1 metal-organic framework crystals. Phys. Rev. B 2007, 76, 184106. [Google Scholar] [CrossRef]
- Chapman, K.W.; Halder, G.J.; Chupas, P.J. Pressure-Induced Amorphization and Porosity Modification in a Metal−Organic Framework. J. Am. Chem. Soc. 2009, 131, 17546–17547. [Google Scholar] [CrossRef]
- Fairen-Jimenez, D.; Moggach, S.A.; Wharmby, T.; Wright, P.A.; Parsons, S.; Düren, S. Opening the Gate: Framework Flexibility in ZIF-8 Explored by Experiments and Simulations. J. Am. Chem. Soc. 2011, 133, 8900–8902. [Google Scholar] [CrossRef]
- Li, W.; Probert, M.R.; Kosa, M.; Bennett, T.D.; Thirumurugan, A.; Burwood, R.P.; Parinello, M.; Howard, J.A.; Cheetham, A.K. Negative Linear Compressibility of a Metal−organic Framework. J. Am. Chem. Soc. 2012, 134, 11940–11943. [Google Scholar] [CrossRef]
- Wu, H.; Yildirim, T.; Zhou, W.J. Exceptional Mechanical Stability of Highly Porous Zirconium Metal-Organic Framework UiO-66 and Its Important Implications. J. Phys. Chem. Lett. 2013, 4, 925–930. [Google Scholar] [CrossRef]
- Ortiz, A.U.; Boutin, A.; Fuchs, A.H.; Coudert, F.X. Anisotropic Elastic Properties of Flexible Metal-Organic Frameworks: How Soft are Soft Porous Crystals? Phys. Rev. Lett. 2012, 109, 195502. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.U.; Boutin, A.; Fuchs, A.H.; Coudert, F.X. Organic Frameworks with Wine-Rack Motif: What Determines their Flexibility and Elastic Properties? J. Chem. Phys. 2013, 138, 174703. [Google Scholar] [CrossRef]
- Ortiz, A.U.; Boutin, A.; Fuchs, A.H.; Coudert, F.X. Investigating the Pressure-Induced Amorphization of Zeolitic Imidazolate Framework ZIF-8: Mechanical Instability Due to Shear Mode Softening. J. Phys. Chem. Lett. 2013, 4, 1861–1865. [Google Scholar] [CrossRef] [PubMed]
- Coudert, F.X. Responsive Metal–Organic Frameworks and Framework Materials: Under Pressure, Taking the Heat, in the Spotlight, with Friends. Chem. Mater. 2015, 27, 1905–1916. [Google Scholar] [CrossRef]
- Cai, W.; Katrusiak, A. Giant negative linear compression positively coupled to massive thermal expansion in a metal–organic framework. Chem. Commun. 2014, 5, 4337–4342. [Google Scholar] [CrossRef]
- Cai, W.; Gładysiak, A.; Anioła, M.; Smith, V.J.; Barbour, L.J.; Katrusiak, A. Giant Negative Area Compressibility Tunable in a Soft Porous Framework Material. J. Am. Chem. Soc. 2015, 137, 9296–9301. [Google Scholar] [CrossRef]
- Serra-Crespo, P.; Dikhtiarenko, A.; Stavitski, E.; Juan-Alcañiz, J.; Kapteijn, F.; Coudert, F.X.; Gascon, J. Experimental evidence of negative linear compressibility in the MIL-53 metal–organic framework family. CrystEngComm 2015, 17, 276–280. [Google Scholar] [CrossRef]
- Tan, J.C.; Bennett, T.D.; Cheetham, A.K. Chemical structure, network topology, and porosity effects on the mechanical properties of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2010, 107, 9938–9943. [Google Scholar] [CrossRef]
- Tan, J.C.; Cheetham, A.K. Mechanical properties of hybrid inorganic–organic framework materials: Establishing fundamental structure–property relationships. Chem. Soc. Rev. 2011, 40, 1059–1080. [Google Scholar] [CrossRef]
- Tan, J.C.; Civalleri, B.; Lin, C.C.; Valenzano, L.; Galvelis, R.; Chen, P.F.; Bennett, T.D.; Mellot-Draznieks, C.; Zicovich-Wilson, C.M.; Cheetham, A.K. Exceptionally Low Shear Modulus in a Prototypical Imidazole-Based Metal-Organic Framework. Phys. Rev. Lett. 2012, 108, 095502. [Google Scholar] [CrossRef] [PubMed]
- Ryder, M.R.; Tan, J.C. Explaining the mechanical mechanisms of zeolitic metal–organic frameworks: Revealing auxeticity and anomalous elasticity. Dalton Trans. 2016, 45, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Yot, P.G.; Boudene, Z.; Macia, J.; Granier, D.; Vanduyfhuys, L.; Verstraelen, T.; Speybroeck, V.V.; Devic, T.; Serre, C.; Ferey, G.; et al. Metal–organic frameworks as potential shock absorbers: The case of the highly flexible MIL-53(Al). Chem. Commun. 2014, 50, 9462–9464. [Google Scholar] [CrossRef] [PubMed]
- Banlusan, K.; Antillon, E.; Strachan, A. Mechanisms of Plastic Deformation of Metal−Organic Framework-5. J. Phys. Chem. C 2015, 119, 25845–25852. [Google Scholar] [CrossRef]
- Banlusan, K.; Amornkitbamrung, V. J Effects of Free Volume on Shock-Wave Energy Absorption in A Metal–Organic Framework: A Molecular Dynamics Investigation. Phys. Chem. C 2020, 124, 17027–17038. [Google Scholar] [CrossRef]
- Fraux, G.; Coudert, F.X.; Boutin, A.; Fuchs, A.H. Forced intrusion of water and aqueous solutions in microporous materials: From fundamental thermodynamics to energy storage devices. Chem. Soc. Rev. 2017, 46, 7421–7437. [Google Scholar] [CrossRef]
- Terracina, A.; Todaro, M.; Mazaj, M.; Agnello, S.; Gelardi, F.M.; Buscarino, G. Unveiled the Source of the Structural Instability of HKUST-1 Powders upon Mechanical Compaction: Definition of a Fully Preserving Tableting Method. J. Phys. Chem. C 2019, 123, 1730–1741. [Google Scholar] [CrossRef]
- Redfern, L.R.; Robison, L.; Wasson, M.C.; Goswami, S.; Lyu, J.; Islamoglu, T.; Chapman, K.W.; Farha, O.K. Isolating the Role of the Node-Linker Bond in the Compression of UiO-66 Metal−Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 4365–4371. [Google Scholar] [CrossRef]
- Moghadam, P.Z.; Rogge, S.M.; Li, A.; Chow, C.M.; Wieme, J.; Moharrami, N.; Aragones-Anglada, M.; Conduit, G.; Gomez-Gualdron, D.A.; Van Speybroeck, V.; et al. Structure-Mechanical Stability Relations of Metal-Organic Frameworks via Machine Learning. Matter 2019, 19, 219–234. [Google Scholar] [CrossRef]
- Zhou, X.; Miao, Y.; Suslick, K.S.; Dlott, D.D. Mechanochemistry of Metal–Organic Frameworks under Pres-sure and Shock. Acc. Chem. Res. 2020, 53, 2806–2815. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, K.; Qiao, Y.; Li, X.; Zou, B. Negative Linear Compressibility Due to Layer Sliding in a Layered Metal−Organic Framework. J. Phys. Chem. Lett. 2017, 8, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Wang, K.; Zou, B. Large Negative Linear Compressibility in InH(BDC)2 from Framework Hinging. J. Am. Chem. Soc. 2017, 139, 15648–15651. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, K.; Zou, B. Negative Linear Compressibility Response to Pressure in Multitype Wine-Rack Metal−Organic Frameworks. ACS Mater. Lett. 2020, 2, 291–295. [Google Scholar] [CrossRef]
- Yan, Y.; O’Connor, A.E.; Kanthasamy, G.; Atkinson, G.; Allan, D.R.; Blake, A.J.; Schroder, M. Unusual and Tunable Negative Linear Compressibility in the Metal-Organic Framework MFM-133(M) (M = Zr, Hf). J. Am. Chem. Soc. 2018, 140, 3952–3958. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Zhang, W.; Dong, L.; Li, W.; Cai, W.; Wei, W.; Ji, L.; Lin, Z.; Lu, P. Negative area compressibility of a hydrogen bonded two-dimensional material. Chem. Sci. 2019, 10, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xu, B.; Li, Q.; Meng, Y.; Quan, Z.; Zou, B. Selected Negative Linear Compressibilities in the Metal−Organic Framework of [Cu(4,4′-bpy)2 (H2O)2]·SiF6. Inorg. Chem. 2020, 59, 1715–1722. [Google Scholar] [CrossRef]
- Zajdel, P.; Chorążewski, M.; Leão, J.B.; Jensen, G.V.; Bleuel, M.; Zhang, H.F.; Feng, T.; Luo, D.; Li, M.; Lowe, A.R.; et al. Inflation Negative Compressibility during Intrusion−Extrusion of a Non-Wetting Liquid into a Flexible Nanoporous Framework. Phys. Chem. Lett. 2021, 12, 4951–4957. [Google Scholar] [CrossRef]
- Tortora, M.; Zajdel, P.; Lowe, A.R.; Chorążewski, M.; Leão, J.B.; Jensen, G.V.; Bleuel, M.; Giacomello, M.; Casciola, C.M.; Meloni, S.; et al. Giant Negative Compressibility by Liquid Intrusion into Superhydrophobic Flexible Nanoporous Frameworks. Nano Lett. 2021, 21, 2848–2853. [Google Scholar] [CrossRef]
- Colmenero, F. Negative Linear Compressibility in Nanoporous Metal–Organic Frameworks Rationalized by the Empty Channel Structural Mechanism. Phys. Chem. Chem. Phys. 2021, 23, 8508–8524. [Google Scholar] [CrossRef]
- Colmenero, F.; Lobato, A.; Timón, V. Compressing the Channels in the Crystal Structure of Copper Squarate Metal-Organic Framework. Solids 2022, 3, 374–384. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, K.; Zou, B. Near Zero Area Compressibility in a Perovskite-Like Metal−Organic Frameworks [C(NH2 )3][Cd(HCOO)3]. ACS Appl. Mater. Interfaces 2018, 10, 23481–23484. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zeng, Q.; Chen, Y.; Jiang, L.; Wang, K.; Zou, B. Extraordinarily Persistent Zero Linear Compressibility in Metal-Organic Framework MIL-122(In). ACS Mater. Lett 2020, 2, 519–523. [Google Scholar] [CrossRef]
- Zhang, X.; Sui, Z.; Xu, B.; Yue, S.; Luo, Y.; Zhan, W.; Liu, B.A. Mechanically strong and highly conductive graphene aerogel and its use as electrodes for electrochemical power source. J. Mater. Chem. 2011, 21, 6494–6497. [Google Scholar] [CrossRef]
- Mecklenburg, M.; Schuchardt, A.; Mishra, Y.K.; Kaps, S.; Adelung, R.; Lotnyk, A.; Kienle, L.; Schulte, K. Aerographite: Ultra Lightweight, Flexible Nanowall, Carbon Microtube Material with Outstanding Mechanical Performance. Adv. Mater. 2012, 24, 3486–3490. [Google Scholar] [CrossRef]
- Worsley, M.A.; Kucheyev, S.O.; Mason, H.E.; Merrill, M.D.; Mayer, B.P.; Lewicki, J.; Valdez, C.A.; Suss, M.E.; Stadermann, M.; Pauzauskie, M.J.; et al. Mechanically robust 3D graphene macroassembly with high surface area. Chem. Commun. 2012, 48, 8428–8430. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhao, Z.; Wan, W.; Gogotsi, Y.; Qiu, J. Ultralight and Highly Compressible Graphene Aerogels. Adv. Mater. 2013, 25, 2219–2223. [Google Scholar] [CrossRef]
- Zhu, C.; Han, T.Y.; Duoss, E.B.; Golobic, A.M.; Kuntz, J.; Spadaccini, C.M.; Worsley, M.A. Highly compressible 3D periodic graphene aerogel microlattices. Nat. Commun. 2015, 6, 6962. [Google Scholar] [CrossRef]
- Lei, J.; Liu, Z. The structural and mechanical properties of graphene aerogels based on Schwarz-surface-like graphene models. Carbon 2018, 130, 741–748. [Google Scholar] [CrossRef]
- Wu, Y.; Yi, N.; Huang, L.; Zhang, T.; Fang, S.; Chang, H.; Li, N.; Oh, J.; Lee, J.A.; Kozlov, M. Three-dimensionally bonded spongy graphene material with super compressive elasticity and near-zero Poisson’s ratio. Nat. Commun. 2015, 6, 6141. [Google Scholar] [CrossRef]
- Chen, B.; Ma, Q.; Tan, C.; Lim, T.T.; Huang, L.; Zhang, H. Carbon-Based Sorbents with Three-Dimensional Architectures for Water Remediation. Small 2015, 11, 3319–3336. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Q.; Yu, Y.; Chen, W.; Hu, H.; Li, H. Naturally Dried Graphene Aerogels with Superelasticity and Tunable Poisson’s Ratio. Adv. Mater. 2016, 28, 9223–9230. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.C.; Mokaya, R. Microporous activated carbon aerogels via a simple subcritical drying route for CO2 capture and hydrogen storage. Microporous Mesoporous Mater. 2013, 179, 151–156. [Google Scholar] [CrossRef]
- Patil, S.P.; Shendye, P.; Markert, B. Molecular investigation of mechanical properties and fracture behavior of graphene aerogel. J. Phys. Chem. B 2020, 124, 6132–6139. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Kulkarni, A.; Markert, B. Shockwave response of graphene aerogels: An all-atom simulation study. Comput. Mater. Sci. 2021, 189, 110252. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, I.D.; Jung, S.M. Multifunctional Inorganic Nanomaterial Aerogel Assembled into fSWNT Hydrogel Platform for Ultraselective NO2 Sensing. ACS Appl. Mater. Interfaces 2020, 12, 10637–10647. [Google Scholar] [CrossRef]
- Barthelet, K.; Marrot, J.; Riou, D.; Férey, G. A Breathing Hybrid Organic-Inorganic Solid with Very Large Pores and High Magnetic Characteristics. Angew. Chem. Int. Ed. 2022, 41, 281–284. [Google Scholar] [CrossRef]
- Bradshaw, D.; Claridge, J.B.; Cussen, E.J.; Prior, T.J.; Rosseinsky, M. Design, Chirality, and Flexibility in Nanoporous Molecule-Based Materials. J. Acc. Chem. Res. 2005, 38, 273–282. [Google Scholar] [CrossRef]
- Serre, C.; Mellot-Draznieks, C.; Surble, C.; Audebrand, N.; Filinchuk, Y.; Ferey, G. Role of Solvent-Host Interactions That Lead to Very Large Swelling of Hybrid Frameworks. Science 2007, 315, 1828–1831. [Google Scholar] [CrossRef]
- Férey, G.; Serre, C. Large breathing effects in three-dimensional porous hybrid matter: Facts, analyses, rules and consequences. Chem. Soc. Rev. 2009, 38, 1380–1399. [Google Scholar] [CrossRef]
- Sato, H.; Kosaka, W.; Matsuda, R.; Hori, A.; Hijikata, Y.; Belosludov, R.V.; Sakaki, S.; Takata, M.; Kitagawa, S. Self-Accelerating CO Sorption in a Soft Nanoporous Crystal. Science 2014, 343, 167–170. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, E.; Mileo, P.G.M.; Sagastuy-Breña, M.; Raziel-Alvarez, J.; Reynolds, J.E.; Villarreal, A.; Gutierrez-Alejandre, A.; Ramirez, J.; Balmaseda, J.; González-Zamora, E.; et al. Highly reversible sorption of H2S and CO2 by an environmentally friendly Mg-based MOF. J. Mater. Chem. A 2018, 6, 16900–16909. [Google Scholar] [CrossRef]
- Remy, T.; Baron, G.V.; Denayer, J.F.M. Modeling the Effect of Structural Changes during Dynamic Separation Processes on MOFs. Langmuir 2011, 27, 13064–13071. [Google Scholar] [CrossRef] [PubMed]
- Remy, T.; Ma, L.; Maes, M.; De Vos, D.E.; Baron, G.V.; Denayer, J.F.M. Vapor-Phase Adsorption and Separation of Ethylbenzene and Styrene on the Metal−Organic Frameworks MIL-47 and MIL-53(Al). Ind. Eng. Chem. Res. 2012, 51, 14824–14833. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Maurin, G.; Ramsahye, N.A.; Balas, F.; Vallet-Regi, M.; Sebban, M.; Taulelle, F.; Férey, G. Flexible Porous Metal-Organic Frameworks for a Controlled Drug Delivery. J. Am. Chem. Soc. 2008, 130, 6774–6780. [Google Scholar] [CrossRef]
- Millange, F.; Serre, C.; Guillou, N.; Férey, G.; Walton, R.I. Structural Effects of Solvents on the Breathing of Metal–Organic Frameworks: An In Situ Diffraction Study. Angew. Chem. Int. Ed. 2008, 47, 4100–4105. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Houk, R.J.T.; Andruszkiewicz, L.; Talin, A.; Pikarsky, J.; Choudhury, A.; Gall, K.A.; Hesketh, P.J. Stress-induced chemical detection using flexible metal-organic frameworks. J. Am. Chem. Soc. 2008, 130, 14404–14405. [Google Scholar] [CrossRef] [PubMed]
- Yot, P.G.; Vanduyfhuys, L.; Alvarez, E.; Rodriguez, J.; Itíe, J.P.; Fabry, P.; Guillou, N.; Devic, T.; Beurroies, I.; Llewellyn, P.L.; et al. Metal−Organic Frameworks as Potential Shock Absorbers: The Case of The Highly Flexible MIL-53(Al). Chem. Sci. 2016, 7, 446–450. [Google Scholar] [CrossRef]
- Baughman, R.H.; Stafstrom, S.; Cui, C.; Dantas, S.O. Compressibilities in One or More Dimensions. Science 1998, 279, 1522–1524. [Google Scholar] [CrossRef]
- Lakes, R.S.; Wojciechowski, K.W. Negative Compressibility, Negative Poisson’s Ratio and Stability. Phys. Stat. Sol. B 2008, 245, 545–551. [Google Scholar] [CrossRef]
- Cairns, A.B.; Goodwin, A.L. Negative Linear Compressibility. Phys. Chem. Chem. Phys. 2015, 17, 20449–20465. [Google Scholar] [CrossRef]
- Xie, Y.M.; Yang, X.; Shen, J.; Yan, X.; Ghaedizadeh, A.; Rong, J.; Huang, X.; Zhou, S. Designing orthotropic materials for negative or zero compressibility. Int. J. Solids Struct. 2014, 51, 4038–4051. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, Y.; Molokeev, M.S.; Gong, L.; Liang, F.; Wang, S.; Liu, L.; Wu, X.; Li, X.; Wu, S.; et al. Zero Linear Compressibility in Nondense Borates with a “Lu-Ban Stool“-Like Structure. Adv. Mater. 2018, 30, 1801313. [Google Scholar] [CrossRef]
- Jiang, X.; Molokeev, M.S.; Dong, L.; Dong, Z.; Wang, N.; Kang, L.; Li, X.; Li, Y.; Tian, C.; Peng, S.; et al. Anomalous mechanical materials squeezing three-dimensional volume compressibility into one dimension. Nat. Commun. 2020, 11, 5593. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dove, M.T.; Shi, J.; Sun, B.; Hu, D.; Wang, D. Adjustable uniaxial zero thermal expansion and zero linear compressibility in unique hybrid semiconductors: The role of the organic chain. Dalton Trans. 2020, 49, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Lakes, R.S. Foam Structures with a Negative Poisson’s Ratio. Science 1987, 235, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, K.W. Constant thermodynamic tension Monte Carlo studies of elastic properties of a two-dimensional system of hard cyclic hexamers. Mol. Phys. 1987, 61, 1247–1258. [Google Scholar] [CrossRef]
- Lakes, R.S. Negative-Poisson’s-Ratio Materials: Auxetic Solids. Annu. Rev. Mater. Res. 2017, 47, 63–81. [Google Scholar] [CrossRef]
- Evans, K.E.; Alderson, A. Auxetic Materials: Functional Materials and Structures from Lateral Thinking! Adv. Mater. 2000, 12, 617–628. [Google Scholar] [CrossRef]
- Spinks, G.M.; Wallace, G.G.; Fifield, L.S.; Dalton, L.R.; Mazzoldi, A.; De Rossi, D.; Khayrullin, I.I.; Baughman, R.H. Pneumatic Carbon Nanotube Actuators. Adv. Mater. 2002, 14, 1728–1732. [Google Scholar] [CrossRef]
- Nicolaou, Z.G.; Motter, A.E. Mechanical Metamaterials with Negative Compressibility Transitions. Nat. Mater. 2012, 11, 608–613. [Google Scholar] [CrossRef]
- Cairns, A.B.; Catafesta, J.; Levelut, C.; Rouquette, J.; Lee, A.; Peters, V.D.; Thompson, A.L.; Dmitriev, V.; Haines, J.; Goodwin, A.L. Giant Negative Linear Compressibility in Zinc Dicyanoaurate. Nat. Mater. 2013, 12, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.; Xi, D.; Xu, J.; Ambati, M.; Srituravanich, W.; Sun, C.; Zhang, X. Ultrasonic metamaterials with negative modulus. Nat. Mater. 2006, 5, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Aliev, A.V.; Oh, J.; Kozlov, M.E.; Kuznetsov, A.A.; Fang, S.; Fonseca, A.F.; Ovalle, R.; Lima, M.D.; Haque, M.H.; Gartstein, Y.N.; et al. Giant-Stroke, Superelastic Carbon Nanotube Aerogel Muscles. Science 2009, 323, 1575–1578. [Google Scholar] [CrossRef]
- Uhoya, W.; Tsoi, G.; Vohra, Y.K.; McGuire, M.A.; Sefat, A.S.; Sales, B.C.; Mandrus, D.; Weir, S.T. nomalous Compressibility Effects and Superconductivity of EuFe2As2 under High Pressures. J. Phys. Cond. Matter. 2010, 22, 292202. [Google Scholar] [CrossRef] [PubMed]
- Alderson, A.; Rasburn, J.; Ameer-Beg, S.; Mullarkey, P.G.; Perrie, W.; Evans, K.E. An auxetic filter: A tuneable filter displaying enhanced size selectivity or defouling properties. Ind. Eng. Chem. Res. 2000, 39, 654–665. [Google Scholar] [CrossRef]
- Alderson, A.; Rasburn, J.; Evans, K.E.; Grima, J.N. Modelling of the mechanical and mass transport properties of auxetic molecular sieves: An idealised organic (polymeric honeycomb) host–guest system. Membr. Technol. 2001, 137, 6–8. [Google Scholar] [CrossRef]
- Rasburn, J.; Alderson, A.; Ameer-Beg, S.; Mullarkey, P.G.; Perrie, W.; Evans, K.E.; Perrie, W.; Evans, K.E. Auxetic structures for variable permeability systems. AIChE J. 2001, 47, 2623–2626. [Google Scholar] [CrossRef]
- Greaves, G.N.; Meneau, F.; Sapelkin, A.; Colyer, L.M.; Gwynn, I.; Wade, S.; Sankar, G. The Rheology of Collapsing Zeolites Amorphized by Temperature and Pressure. Nat. Mater. 2003, 2, 622–628. [Google Scholar] [CrossRef]
- Haines, J.; Levelut, C.; Isambert, A.; Hebert, P.; Kohara, S.; Keen, D.A.; Hamouda, T.; Andraul, D. Topologically Ordered Amorphous Silica Obtained from the Collapsed Siliceous Zeolite, Silicalite-1-F, A Step toward Perfect Glasses. J. Am. Chem. Soc. 2009, 131, 12333–12338. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Chen, L.; Shen, Z. Formation of a unique glass by spark plasma sintering of a zeolite. J. Mater. Res. 2009, 24, 3241–3245. [Google Scholar] [CrossRef]
- Moggach, S.A.; Bennett, T.D.; Cheetham, A.K. The Effect of Pressure on ZIF-8: Increasing Pore Size with Pressure and the Formation of a High-Pressure Phase at 1.47 GPa. Angew. Chem. 2009, 121, 7221–7223. [Google Scholar] [CrossRef]
- Hwang, G.C.; Shin, T.J.; Blom, D.A.; Vogt, T.; Lee, Y. Pressure-Induced Amorphization of Small Pore Zeolites—The Role of Cation-H2O Topology and Antiglass Formation. Sci. Rep. 2015, 5, 15056. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Gorelli, F.; Haines, J.; Cambon, O.; Levelut, C.; Garbarino, G. Silicon carbonate phase formed from carbon dioxide and silica under pressure. Proc. Natl. Acad. Sci. USA 2011, 108, 7689–7692. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Gorelli, F.A.; Bini, R. Carbon enters silica forming a cristobalite-type CO2-SiO2 solid solution. Nat. Commun. 2013, 4, 1557. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Gorelli, F.; Bini, R.; Salamat, A.; Garbarino, G.; Levelut, C.; Cambon, O.; Haines, J. High-Pressure Synthesis of a Polyethylene/Zeolite Nano-Composite Material. Nat. Commun. 2014, 5, 3761. [Google Scholar] [CrossRef]
- Jorda, J.F.; Rey, F.; Sastre, G.; Valencia, S.; Palomino, M.; Corma, A.; Segura, A.; Errandonea, D.; Lacomba, R.; Manjon, F.J.; et al. Synthesis of a Novel Zeolite through a Pressure-Induced Reconstructive Phase Transition Process. Angew. Chem. Int. Ed. 2013, 125, 10652–10656. [Google Scholar] [CrossRef]
- Zhou, R.; Qu, B.; Dai, J.; Zeng, X.C. Unravelling the crystal structure of the high-pressure phase of silicon carbonate. Phys. Rev. X 2014, 4, 011030. [Google Scholar]
- Marques, M.; Morales-Garcia, A.; Menendez, J.M.; Baonza, V.G.; Recio, J.M. A novel crystalline SiCO compound. Phys. Chem. Chem. Phys. 2015, 17, 25055–25060. [Google Scholar] [CrossRef]
- Santamaria-Perez, D.; Marqueño, T.; MacLeod, S.; Ruiz-Fuertes, J.; Daisenberger, D.; Chuliá-Jordan, R.; Errandonea, D.; Jordá, J.L.; Rey, F.; McGuire, C.; et al. Structural Evolution of CO2-Filled Pure Silica LTA Zeolite under High- Pressure High-Temperature Conditions. Chem. Mater. 2017, 29, 4502–4510. [Google Scholar] [CrossRef]
- Marqueño, T.; Santamaria-Perez, D.; Ruiz-Fuertes, J.; Chuliá-Jordán, R.; Jordá, J.L.; Rey, F.; McGuire, C.; Kavner, A.; MacLeod, A.S.; Daisenberger, D.; et al. An Ultrahigh CO2-Loaded Silicalite-1 Zeolite: Structural Stability and Physical Properties at High Pressures and Temperatures. Inorg. Chem. 2018, 57, 6447–6455. [Google Scholar] [CrossRef]
- Thibaud, J.M.; Rouquette, J.; Hermet, P.; Dziubek, K.; Gorelli, M.; Santoro, M.; Garbarino, G.; Alabarse, F.G.; Cambon, O.; Di Renzo, F.; et al. Saturation of the Siliceous Zeolite TON with Neon at High Pressure. J. Phys. Chem. C 2017, 121, 4283–4292. [Google Scholar] [CrossRef]
- Tan, C.; Liu, Z.; Yonezawa, Y.; Sukenaga, S.; Ando, M.; Shibata, H.; Sasaki, Y.; Okubo, T.; Wakihara, T. Unique Crystallization Behavior in Zeolite Synthesis under External High Pressures. Chem. Commun. 2020, 56, 2811–2814. [Google Scholar] [CrossRef]
- Deneyer, A.; Ke, J.; Devos, M.; Dusselier, M. Zeolite Synthesis under Nonconventional Conditions: Reagents, Reactors, and Modi Operandi. Chem. Mater. 2020, 32, 4884–4919. [Google Scholar] [CrossRef]
- Lv, H.; Yao, M.; Li, Q.; Liu, R.; Liu, B.; Lu, S.; Jiang, L.; Cui, W.; Liu, Z.; Liu, J.; et al. The structural stability of AlPO4-5 zeolite under pressure: Effect of the pressure transmission medium. J. Appl. Phys. 2012, 111, 112615. [Google Scholar] [CrossRef]
- Kim, T.; Lee, Y.; Jang, Y.N.; Shin, J.; Hong, S.B. Contrasting high-pressure compression behaviors of AlPO4-5 and SSZ-24 with the same AFI framework topology. Microporous Mesoporous Mater. 2013, 169, 42–46. [Google Scholar] [CrossRef]
- Lotti, P.; Gatta, G.D.; Comboni, D.; Merlini, M.; Pastero, L.; Hanfland, M. AlPO4-5 zeolite at high pressure: Crystal-fluid interaction and elastic behavior. Microporous Mesoporous Mater. 2016, 228, 158–167. [Google Scholar] [CrossRef]
- Alabarse, F.G.; Silly, G.; Haidoux, A.; Levelut, C.; Bourgogne, D.; Flank, A.M.; Lagarde, P.; Pereira, A.S.; Bantignies, J.L.; Cambon, O.; et al. Effect of H2O on the Pressure-Induced Amorphization of AlPO4-54·xH2O. Phys. Chem. C 2014, 118, 3651–3663. [Google Scholar] [CrossRef]
- Alabarse, F.G.; Brubach, J.; Roy, P.; Haidoux, A.; Levelut, C.; Bantignies, J.L.; Cambon, O.; Haines, J. AlPO4-54 − AlPO4-8 Structural Phase Transition and Amorphization under High Pressure. Mechanism of H2O Insertion and Chemical Bond Formation in AlPO4-54·xH2O at High Pressure. J. Phys. Chem. C 2015, 119, 7771–7779. [Google Scholar] [CrossRef]
- Alabarse, F.G.; Rouquette, J.; Coasne, B.; Haidoux, A.; Paulmann, C.; Cambon, O.; Haines, J. Mechanism of H2O insertion and chemical bond formation in AlPO(4)-54·xH2O at high pressure. J. Am. Chem. Soc. 2015, 137, 584–587. [Google Scholar] [CrossRef]
- Alabarse, F.G.; Silly, G.; Brubach, J.B.; Roy, P.; Haidoux, A.; Levelut, C.; Bantignies, J.B.; Kohara, S.; Floch, S.L.; Cambon, O.; et al. Anomalous Compressibility and Amorphization in AlPO4-17, the Oxide with the Highest Negative Thermal Expansion. Phys. Chem. C 2017, 121, 6852–6863. [Google Scholar] [CrossRef]
- Alabarse, F.G.; Joseph, B.; Lausi, A.; Haines, J. Effect of H2O on the Pressure-Induced Amorphization of Hydrated AlPO4-17. Molecules 2019, 24, 2864. [Google Scholar] [CrossRef] [PubMed]
- Domenico, S.N. Elastic properties of unconsolidated porous sand reservoirs. Geophysics 1977, 42, 1339–1368. [Google Scholar] [CrossRef]
- Eberhart-Phillips, D.; Han, D.; Zoback, M. Empirical relationships among seismic velocity, effective pressure, porosity, and clay content in sandstone. Geophysics 1989, 54, 82–89. [Google Scholar] [CrossRef]
- Thomsen, L. Elastic Anisotropy Due to Aligned Cracks in Porous Rock. Geophys. Prospect. 1995, 3, 805–829. [Google Scholar] [CrossRef]
- Dvorkin, J.; Nur, A. Elasticity of high-porosity sandstones: Theory for two North Sea data sets. Geophysics 1996, 61, 1363–1370. [Google Scholar] [CrossRef]
- Nur, A.; Mavko, G.; Dvorkin, J.; Galmudi, D. Diagnosing high-porosity sandstones: Strength and permeability from porosity and velocity. Lead. Edge 1998, 17, 357–362. [Google Scholar] [CrossRef]
- Schön, J.H. Chapter 6 Elastic Properties. In Physical Properties of Rocks, A Workbook. Handbook of Petroleum Exploration and Production; Schön, J.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume, 8, pp. 149–243. [Google Scholar]
- Alonso, E.E.; Vaunat, J.; Gens, A. Modelling the mechanical behaviour of expansive clays. Eng. Geol. 1999, 54, 173–183. [Google Scholar] [CrossRef]
- Åkesson, A.; Kristensson, O. Mechanical modeling of MX-80–Development of constitutive laws. Phys. Chem. Earth 2008, 33, S504–S507. [Google Scholar] [CrossRef]
- Tisato, N.; Marelli, S. Laboratory measurements of the longitudinal and transverse wave velocities of compacted bentonite as a function of water content, temperature, and confining pressure. J. Geophys. Res. Solid Earth 2013, 118, 3380–3393. [Google Scholar] [CrossRef]
- Keller, L.M. Porosity anisotropy of Opalinus Clay: Implications for the poroelastic behaviour. Geophys. J. Int. 2017, 208, 1443–1448. [Google Scholar] [CrossRef]
- Kenigsberg, A.R.; Rivière, J.; Marone, C.; Saffer, D.M. Evolution of Elastic and Mechanical Properties during Fault Shear: The Roles of Clay Content, Fabric Development, and Porosity. J. Geophys. Res. Solid Earth 2019, 124, 10968–10982. [Google Scholar] [CrossRef]
- Kenigsberg, A.R.; Rivière, J.; Marone, C.; Saffer, D.M. The Effects of Shear Strain, Fabric, and Porosity Evolution on Elastic and Mechanical Properties of Clay-Rich Fault Gouge. J. Geophys. Res. Solid Earth 2020, 125, e2019JB018612. [Google Scholar] [CrossRef]
- Liu, K.; Sheng, J.; Zhang, Z. A simulation study of the effect of clay swelling on fracture generation and porosity change in shales under stress anisotropy. Eng. Geol. 2020, 278, 105829. [Google Scholar] [CrossRef]
- Sveinsson, H.A.; Ning, F.; Cao, P.; Fang, B.; Malthe-Sørenssen, A. Grain-Size-Governed Shear Failure Mechanism of Polycrystalline Methane Hydrates. J. Phys. Chem. C 2021, 125, 10034–10042. [Google Scholar] [CrossRef]
- Yu, C.; Ji, S.; Li, Q. Effects of porosity on seismic velocities, elastic moduli and Poisson’s ratios of solid materials and rocks. J. Rock Mech. Geotech. Eng. 2016, 8, 35–49. [Google Scholar] [CrossRef]
- Brantut, N.; Stefanou, I.; Sulem, J. Dehydration-induced instabilities at intermediate depths in subduction zones. J. Geophys. Res. Solid Earth 2017, 122, 6087–6107. [Google Scholar] [CrossRef]
- Born, M. On the Stability of Crystal Lattices. Math. Proc. Camb. Phil. Soc. 1940, 36, 160–172. [Google Scholar] [CrossRef]
- Milstein, F. Theoretical elastic behaviour of crystals at large strains. J. Mater. Sci. 1980, 15, 1071–1084. [Google Scholar] [CrossRef]
- Karki, B.B.; Ackland, G.J.; Crain, J. Elastic instabilities in crystals from ab initio stress–strain relations. J. Phys. Condens. Matter 1997, 9, 8579–8589. [Google Scholar] [CrossRef]
- Mouhat, F.; Coudert, F.X. Necessary and Sufficient Elastic Stability Conditions in Various Crystal Systems. Phys. Rev. B 2014, 90, 224104. [Google Scholar] [CrossRef]
- Gao, F. Hardness estimation of complex oxide materials. Phys. Rev. B 2004, 69, 094113. [Google Scholar] [CrossRef]
- Šimůnek, A.; Vackář, J. Hardness of Covalent and Ionic Crystals: First-Principle Calculations. Phys. Rev. Lett. 2006, 96, 085501. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Wei, P.; Sun, Y.; Chen, C.X.; Franchini, C.; Li, D.; Li, Y. Electronic, optical, and mechanical properties of superhard cold-compressed phases of carbon. Appl. Phys. Lett. 2011, 99, 031901. [Google Scholar] [CrossRef]
- Chen, X.Q.; Niu, H.; Li, D.; Li, Y. Modeling Hardness of Polycrystalline Materials and Bulk Metallic Glasses. Intermetallics 2011, 19, 1275–1281. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Wang, W.; Fu, Z. Simple Method for the Hardness Estimation of Inorganic Crystals by the Bond Valence Model. Inorg. Chem. 2016, 55, 11089–11095. [Google Scholar] [CrossRef]
- Pugh, S.F. Relations between the Elastic Moduli and the Plastic Properties of Polycrystalline Pure Metals. Philos. Mag. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Pettifor, D.G. Theoretical predictions of structure and related properties of intermetallics. Mater. Sci. Technol. 1992, 8, 345–349. [Google Scholar] [CrossRef]
- Niu, H.; Chen, X.Q.; Liu, P.; Xing, W.; Cheng, X.; Li, D.; Li, Y. Extra-electron induced covalent strengthening and generalization of intrinsic ductile-to-brittle criterion. Sci. Rep. 2012, 2, 718. [Google Scholar] [CrossRef]
- Bouhadda, Y.; Djella, S.; Bououdina, M.; Fenineche, N.; Boudouma, Y. Structural and Elastic Properties of LiBH4 for Hydrogen Storage Applications. J. Alloys Compd. 2012, 534, 20–24. [Google Scholar] [CrossRef]
- Gschneidner, K.; Russell, A.; Pecharsky, A.; Morris, J.; Zhang, Z.; Lograsso, T.; Hsu, D.; Lo, H.C.; Ye, Y.; Slager, A.; et al. A family of ductile intermetallic compounds. Nat. Mater. 2003, 2, 587–591. [Google Scholar] [CrossRef]
- Ritchie, R.O. The conflicts between strength and toughness. Nat. Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Cuddy, E.; Lin, J.; Kaufman, J.L.; Shaw, A.; Conway, P.L.J.; Pribram-Jones, A.; Laws, K.J.; Bassman, L. Predicting ductility in quaternary -like alloys. Phys. Rev. Mater. 2021, 5, 033604. [Google Scholar] [CrossRef]
- Zener, C. Elasticity and Anelasticity of Metals; University of Chicago Press: Chicago, IL, USA, 1948. [Google Scholar]
- Chung, D.H.; Buessem, W.R. The Elastic Anisotropy of Crystals. J. Appl. Phys. 1967, 38, 2010–2012. [Google Scholar] [CrossRef]
- Tvergaard, V.; Hutchinson, J.H. Microcracking in ceramics induced by thermal expansion or elastic anisotropy. J. Am. Ceram. Soc. 1988, 71, 157–166. [Google Scholar] [CrossRef]
- Ravindran, P.; Fast, L.; Korzhavyi, P.A.; Johansson, B.; Wills, J.; Eriksson, O. Density Functional Theory for Calculation of Elastic Properties of Orthorhombic Crystals: Application to TiSi2. J. Appl. Phys. 1997, 84, 4891–4904. [Google Scholar] [CrossRef]
- Ledbetter, H.; Migliori, A.A. A general elastic-anisotropy measure. J. Appl. Phys. 2006, 100, 063516. [Google Scholar] [CrossRef]
- Lloveras, P.; Castan, T.; Porta, M.; Planes, A.; Saxena, A. Influence of Elastic Anisotropy on Structural Nanoscale Textures. Phys. Rev. Lett. 2008, 100, 165707. [Google Scholar] [CrossRef]
- Kube, C.M. Elastic anisotropy of crystals. AIP Adv. 2016, 6, 095209. [Google Scholar] [CrossRef]
- Ranganathan, S.I.; Ostoja-Starzewski, M. Universal Elastic Anisotropy Index. Phys. Rev. Lett. 2008, 101, 055504. [Google Scholar] [CrossRef]
- Curtin, W.A. Theory of Mechanical Properties of Ceramic-Matrix Composites. J. Am. Ceram. Soc. 1991, 74, 2837–2845. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’ko, Y.K. Small but strong: A review of the mechanical properties of carbon nanotube–polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Salvetat, J.P.; Briggs, G.A.D.; Bonard, J.M.; Bacsa, R.R.; Kulik, A.J.; Stöckli, T.; Burnham, N.A.; Forró, L. lastic and Shear Moduli of Single-Walled Carbon Nanotube Ropes. Phys. Rev. Lett. 1999, 82, 944–947. [Google Scholar] [CrossRef]
- Kis, A.; Csányi, G.; Salvetat, J.P.; Lee, T.N.; Couteau, E.; Kulik, A.J.; Benoit, W.; Brugger, J.; Forró, L. Reinforcement of single-walled carbon nanotube bundles by intertube bridging. Nat. Mater. 2004, 3, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Beyer, T.; Day, G.M.; Price, S.L. The prediction, morphology, and mechanical properties of the polymorphs of paracetamol. J. Am. Chem. Soc. 2001, 123, 5086–5094. [Google Scholar] [PubMed]
- Reddy, C.M.; Basavoju, S.; Desiraju, G.R. Structural basis for bending of organic crystals. Chem. Commun. 2005, 2005, 2439–2441. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.M.; Krishna, G.R.; Ghosh, S. Mechanical properties of molecular crystals—Applications to crystal engineering. CrystEngComm 2010, 12, 2296–2314. [Google Scholar] [CrossRef]
- Raju, K.B.; Ranjan, S.; Vishnu, V.S.; Bhattacharya, M.; Bhattacharya, B.; Mukhopadhyay, A.K.; Reddy, C.M. Rationalizing Distinct Mechanical Properties of Three Polymorphs of a Drug Adduct by Nanoindentation and Energy Frameworks Analysis: Role of Slip Layer Topology and Weak Interactions. Cryst. Growth Des. 2018, 8, 3927–3937. [Google Scholar] [CrossRef]
- Fabbiani, F.P.A.; Pulham, C.R. High-pressure studies of pharmaceutical compounds and energetic materials. Chem. Soc. Rev. 2006, 35, 932–942. [Google Scholar] [CrossRef]
- Fabbiani, F.P.A.; Allan, D.R.; David, W.I.F.; Davidson, A.J.; Lennie, A.R.; Parsons, S.; Pulham, C.R.; Warren, J.E. High-pressure studies of pharmaceuticals: An exploration of the behavior of piracetam. Cryst. Growth Des. 2007, 7, 1115–1124. [Google Scholar] [CrossRef]
- Neumann, M.A.; Van de Streek, J.; Fabbiani, F.P.A.; Hidber, P.; Grassmann, O. Combined crystal structure prediction and high-pressure crystallization in rational pharmaceutical polymorph screening. Nat. Commun. 2015, 6, 7793. [Google Scholar] [CrossRef]
- Meier, M.; John, E.; Wieckhusen, D.; Wirth, W.; Peukert, W. Influence of mechanical properties on impact fracture: Prediction of the milling behaviour of pharmaceutical powders by nanoindentation. Powder Technol. 2009, 188, 301–313. [Google Scholar] [CrossRef]
- Karki, S.; Friščić, T.; Fábián, L.; Laity, P.R.; Day, G.M.; Jones, W. Improving Mechanical Properties of Crystalline Solids by Cocrystal Formation: New Compressible Forms of Paracetamol. Adv. Mater. 2009, 21, 3905–3909. [Google Scholar] [CrossRef]
- Varughese, S.; Kiran, M.S.R.N.; Solanko, K.A.; Bond, A.D.; Ramamurty, U.; Desiraju, G.R. Interaction anisotropy and shear instability of aspirin polymorphs established by nanoindentation. Chem. Sci. 2011, 2, 2236–2242. [Google Scholar] [CrossRef]
- SeethaLekshmi, S.; Kiran, M.S.R.N.; Ramamurty, U.; Varughese, S. Molecular basis for the mechanical response of sulfa drug crystals. Chem. Eur. J. 2019, 25, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Egar, M.; Janković, B.; Lah, N.; Ilić, I.; Srčič, S. Nanomechanical properties of selected single pharmaceutical crystals as a predictor of their bulk behaviour. Pharm. Res. 2015, 3, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Azuri, I.; Meirzadeh, E.; Here, D.; Cohen, S.R.; Rappe, A.; Lahav, M.; Lubomirsky, I.; Kronik, L. Unusually Large Young’s Moduli of Amino Acid Molecular Crystals. Angew. Chem. Int. Ed. 2015, 54, 13566–13570. [Google Scholar] [CrossRef]
- Rupasinghe, T.M.; Hutchins, K.M.; Bandaranayake, B.S.; Ghorai, S.; Karunatilake, C.; Bučar, D.K.; Swenson, D.C.; Arnold, M.A.; MacGillivray, L.R.; Tivanski, A.V. Mechanical Properties of a Series of Macro- and Nanodimensional Organic Cocrystals Correlate with Atomic Polarizability. J. Am. Chem. Soc. 2015, 137, 12768–12771. [Google Scholar] [CrossRef]
- Mohamed, M.R.; Mishra, M.K.; Al Harbi, L.M.; Al Ghamdic, M.S.; Ramamurty, U. Anisotropy in the mechanical properties of organic crystals: Temperature dependence. RSC Adv. 2015, 5, 64156–64162. [Google Scholar] [CrossRef]
- Mishra, M.K.; Mishra, K.; Narayan, A.; Reddy, C.M.; Vangala, V.R. Structural Basis for Mechanical Anisotropy in Polymorphs of a Caffeine–Glutaric Acid Cocrystal. Cryst. Growth Des. 2020, 20, 6306–6315. [Google Scholar] [CrossRef]
- Mishra, M.K.; Sun, C.C. Conformation Directed Interaction Anisotropy Leading to Distinct Bending Behaviors of Two ROY Polymorphs. Cryst. Growth Des. 2020, 20, 4764–4769. [Google Scholar] [CrossRef]
- Jain, A.; Shah, H.S.; Johnson, P.R.; Narang, A.S.; Morris, K.R.; Haware, R.V. Crystal anisotropy explains structure-mechanics impact on tableting performance of flufenamic acid polymorphs. Eur. J. Pharm. Biopharm. 2018, 132, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Singaraju, A.B.; Bahl, D.; Wang, C.; Swenson, D.C.; Sun, C.C.; Stevens, L.L. Molecular Interpretation of the Compaction Performance and Mechanical Properties of Caffeine Cocrystals: A Polymorphic Study. Mol. Pharmaceutics 2020, 17, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Sun, C.C.; Liu, Y.; Wang, C.; Shi, P.; Xu, J.; Wu, S.; Gong, J. Fabrication of a microcapsule extinguishing agent with a core–shell structure for lithium-ion battery fire safety. Chem. Mater. 2021, 33, 1053–1060. [Google Scholar] [CrossRef]
- Vaksler, Y.; Idrissi, A.; Urzhuntseva, V.V.; Shishkina, S.V. Quantum Chemical Modeling of Mechanical Properties of Aspirin Polymorphic Modifications. Cryst. Growth Des. 2021, 21, 2176–2186. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Wu, S.; Fan, Y.; Li, X. Influence of the mechanical properties of biomaterials on degradability, cell behaviors and signaling pathways: Current progress and challenges. Biomater. Sci. 2020, 8, 2714–2733. [Google Scholar] [CrossRef]
- Mitragotri, M.; Llahann, J. Physical approaches to biomaterial design. Nat. Mater. 2009, 8, 15–23. [Google Scholar] [CrossRef]
- Jahan, A.; Ismail, M.Y.; Sapuan, S.; Mustapha, F. Material screening and choosing methods—A review. Mater. Des. 2010, 31, 696–705. [Google Scholar] [CrossRef]
- Li, Z.; Nevitt, M.N.; Ghose, S. Elastic constants of sodalite Na4Al3Si3O12Cl. Appl. Phys. Lett. 1989, 55, 1730–1731. [Google Scholar] [CrossRef]
- Lethbridge, Z.A.D.; Walton, R.I.; Bosak, A.; Krisch, M. Single-crystal elastic constants of the zeolite analcime measured by inelastic X-ray scattering. Chem. Phys. Lett. 2009, 471, 286–289. [Google Scholar] [CrossRef]
- Williams, J.J.; Evans, K.E.; Walton, R.I. On the elastic constants of the zeolite chlorosodalite. Appl. Phys. Lett. 2006, 88, 021914. [Google Scholar] [CrossRef]
- Birch, F. The Velocity of Compressional Waves in Rocks to 10 Kilobars, Part 1. J. Geophys Res. 1960, 65, 1083–1102. [Google Scholar] [CrossRef]
- Neighbours, J.R.; Schacher, G.E. Determination of Elastic Constants from Sound-Velocity Measurements in Crystals of General Symmetry. J. Appl. Phys. 1967, 38, 5366–5375. [Google Scholar] [CrossRef]
- Christensen, N.I. Poisson′s ratio and crustal seismology. J. Geophys. Res. 1996, 101, 3139–3156. [Google Scholar] [CrossRef]
- Li, B.; Liebermann, R.C. Study of the Earth’s Interior Using Measurements of Sound Velocities in Minerals by Ultrasonic Interferometry. Phys. Earth Planet. Int. 2014, 233, 135–153. [Google Scholar] [CrossRef]
- Bosak, A.; Serrano, J.; Krisch, M.; Watanabe, K.; Taniguchi, T.; Kanda, H. Elasticity of hexagonal boron nitride: Inelastic x-ray scattering measurements. Phys. Rev. B 2006, 73, 041402. [Google Scholar] [CrossRef]
- Bosak, A.; Krisch, M.; Mohr, M.; Maultzsch, J.; Thomsen, C. Elasticity of single-crystalline graphite: Inelastic x-ray scattering study. Phys. Rev. B 2007, 75, 153408. [Google Scholar] [CrossRef]
- Diddens, I.; Murphy, B.; Krisch, M.; Müller, M. Anisotropic Elastic Properties of Cellulose Measured Using Inelastic X-ray Scattering. Macromolecules 2008, 41, 9755–9759. [Google Scholar] [CrossRef]
- Kiefte, H.3; Breckon, S.; Penney, R.; Clouter, M. Elastic constants of ammonia by Brillouin spectroscopy. J. Chem. Phys. 1985, 83, 4738–4743. [Google Scholar] [CrossRef]
- Bass, J.D. Elasticity of grossular and spessartite garnets by Brillouin sepctroscopy. J. Geophys. Res. 1989, 94, 7621–7628. [Google Scholar] [CrossRef]
- Bezacier, L.; Reynard, B.; Cardon, H.; Montagnac, G.; Bass, J.D. High-pressure elasticity of serpentine and seismic properties of the hydrated mantle wedge. J. Geophys. Res. Solid Earth 2013, 118, 527–535. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Lin, J.; Shu, X.F.; Dong, J.X. The synthesis and mechanical properties of large zeolite sodalite single crystals. Stud. Surf. Sci. Catal. A 2005, 158, 231–238. [Google Scholar]
- Brabec, L.; Bohac, P.; Stranyanek, M.; Ctvrtlik, R.; Kocirik, M. Hardness and elastic modulus of silicalite-1 crystal twins. Microporous Mesoporous Mater. 2006, 94, 226–233. [Google Scholar] [CrossRef]
- Johnson, M.C.; Wang, J.; Li, Z.; Lew, C.M.; Yan, Y. Effect of calcination and polycrystallinity on mechanical properties of nanoporous MFI zeolites. Mater. Sci. Eng. A 2007, 456, 58–63. [Google Scholar] [CrossRef]
- Eslava, S.; Zhang, L.; Esconjauregui, S.; Yang, J.; Baklanov, K.M.; Saiz, M.R. Metal-organic framework ZIF-8 films as low-κ dielectrics in microelectronics. Chem. Mater. 2013, 25, 27–33. [Google Scholar] [CrossRef]
- Zeng, Z.; Tan, J.C. AFM Nanoindentation to Quantify Mechanical Properties of Nano- and Micron-Sized Crystals of a Metal-Organic Framework Material. ACS Appl. Mater. Interfaces 2017, 9, 39839–39854. [Google Scholar] [CrossRef]
- Boissiere, C.; Grosso, D.; Lepoutre, S.; Nicole, L.; Bruneau, A.B.; Sanchez, C. Porosity and mechanical properties of mesoporous thin films assessed by environmental ellipsometric porosimetry. Langmuir 2005, 21, 12362–12371. [Google Scholar] [CrossRef] [PubMed]
- Devine, S.D.; Robinson, W.H. Piezoelectric method of determining mechanical properties of a small sandwich specimen at 30 to 200 kHz. J. Appl. Phys. 1977, 48, 1437. [Google Scholar] [CrossRef]
- Woldenden, A.; Harmouche, M.R. Elastic constants of silver as a function of temperature. J. Mater. Sci. 1993, 28, 1015–1018. [Google Scholar] [CrossRef]
- Ganesan, V.V.; Dhanasekaran, M.; Thangavel, N.; Dhathathreyan, A. Elastic compliance of fibrillar assemblies in type I collagen. Biophys. Chem. 2018, 240, 15–24. [Google Scholar] [CrossRef]
- Kiely, E.; Zwane, R.; Fox, R.; Reilly, A.M.; Guerin, S. Density functional theory predictions of the mechanical properties of crystalline materials. CrystEngComm 2021, 23, 5697–5710. [Google Scholar] [CrossRef]
- Chaudhuri, M.M. The deformation stress of highly brittle explosive crystals from real contact area measurements. J. Mater. Sci. 1984, 19, 3028–3042. [Google Scholar] [CrossRef]
- Wang, Z.; Lambros, J.; Lobo, R.F. Micromechanical compressive response of a zeolite single crystal. J. Mater. Sci. 2002, 37, 2491–2499. [Google Scholar] [CrossRef]
- Singh, A.K. X-ray diffraction from solids under nonhydrostatic compression—Some recent studies. J. Phys. Chem. Solids 2004, 65, 1589–1596. [Google Scholar] [CrossRef]
- Singh, A.K.; Andrault, D.; Bouvier, P. X-ray diffraction from stishovite under nonhydrostatic compression to 70 GPa: Strength and elasticity across the tetragonal → orthorhombic transition. Phys. Earth Planet. Inter. 2012, 208–209, 1–10. [Google Scholar] [CrossRef]
- Duwal, S.; Yoo, C.S. Shear-Induced Isostructural Phase Transition and Metallization of Layered Tungsten Disulfide under Nonhydrostatic Compression. J. Phys. Chem. C 2016, 120, 5101–5107. [Google Scholar] [CrossRef]
- Liu, B.; Lin, L.; Gao, Y.; Ma, Y.; Zhou, P.; Han, D.; Gao, C. Metallization of Molybdenum Diselenide under Nonhydrostatic Compression. J. Phys. Chem. C 2021, 125, 5412–5416. [Google Scholar] [CrossRef]
- Day, G.M.; Price, S.L.; Leslie, M. Elastic Constant Calculations for Molecular Organic Crystals. Cryst. Growth Des. 2001, 1, 13–27. [Google Scholar] [CrossRef]
- Han, S.S.; Goddard, W.A., III. Metal-Organic Frameworks Provide Large Negative Thermal Expansion Behavior. J. Phys. Chem. C 2007, 11, 15185–15191. [Google Scholar] [CrossRef]
- Wan, C.; Sun, C.C. Superior Plasticity and Tabletability of Theophylline Monohydrate. Mol. Pharm. 2019, 16, 1732–1741. [Google Scholar]
- Stixrude, L.; Cohen, R.E.; Singh, D.J. Iron at high pressure: Linearized-augmented-plane-wave computations in the generalized-gradient approximation. Phys. Rev. B 1994, 50, 6442–6445. [Google Scholar] [CrossRef] [PubMed]
- Karki, B.; Stixrude, L.; Wentzcovitch, R.M. High-pressure elastic properties of major materials of Earth’s mantle from first principles. Rev. Geophys. 2001, 39, 507–534. [Google Scholar] [CrossRef]
- De Jong, M.; Chen, W.; Angsten, T.; Jain, A.; Notestine, R.; Gamst, A.; Sluiter, M.; Ande, C.K.; van der Zwaag, S.; Plata, J.J.; et al. Charting the complete elastic properties of inorganic crystalline compounds. Sci. Data 2015, 2, 150009. [Google Scholar] [CrossRef] [PubMed]
- Chibani, S.; Coudert, F.X. Systematic exploration of the mechanical properties of 13 621 inorganic compounds. Chem. Sci. 2019, 10, 8589–8599. [Google Scholar] [CrossRef]
- Payne, M.C.; Teter, M.P.; Ailan, D.C.; Arias, A.; Joannopoulos, J.D. terative Minimization Techniques for ab Initio Total-Energy Calculations: Molecular Dynamics and Conjugate Gradients. Rev. Mod. Phys. 1992, 64, 1045–1097. [Google Scholar] [CrossRef]
- MaterialsStudio. Available online: https://3dsbiovia.com/products/collabo-rative-science/biovia-materials-studio/ (accessed on 15 June 2021).
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ruzsinszky, A.; Csonka, G.I.; Vydrov, O.A.; Scuseria, G.E.; Constantin, L.A.; Zhou, X.; Burke, K. Restoring the Density-Gradient Expansion for Exchange in Solids and Surfaces. Phys. Rev. Lett. 2008, 100, 136406. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type Density Functional Constructed with a Long-Range Dispersion Correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Bonales, L.J.; Colmenero, F.; Cobos, J.; Timón, V. Spectroscopic Raman characterization of rutherfordine: A combined DFT and experimental study. Phys. Chem. Chem. Phys. 2016, 18, 16575–16584. [Google Scholar] [CrossRef]
- Colmenero, F.; Bonales, L.J.; Cobos, J.; Timón, V. Density Functional Theory Study of the Thermodynamic and Raman Vibrational Properties of γ-UO3 Polymorph. J. Phys. Chem. C 2017, 121, 14507–14516. [Google Scholar] [CrossRef]
- Colmenero, F.; Bonales, L.J.; Cobos, J.; Timón, V. Thermodynamic and Mechanical Properties of the Rutherfordine Mineral Based on Density Functional Theory.J. Phys. Chem. C 2017, 121, 5994–6001. [Google Scholar] [CrossRef]
- Weck, P.F.; Gordon, M.; Greathouse, J.A.; Bryan, C.E.; Meserole, S.P.; Rodriguez, M.A.; Payne, M.C.; Kim, E.J. Infrared and Raman Spectroscopy of α-ZrW2O8: A Comprehensive Density Functional Perturbation Theory and Experimental Study. J. Raman Spectrosc. 2018, 49, 1373–1384. [Google Scholar] [CrossRef]
- Colmenero, F.; Plášil, J.; Sejkora, J. The crystal structures and mechanical properties of the uranyl carbonate minerals roubaultite, fontanite, sharpite, widenmannite, grimselite and čejkaite. Inorg. Chem. Front. 2020, 7, 4197–4221. [Google Scholar] [CrossRef]
- Colmenero, F. Thermodynamic properties of the uranyl carbonate minerals roubaultite, fontanite, widenmannite, grimselite, čejkaite and bayleyite. Inorg. Chem. Front. 2020, 7, 4160–4179. [Google Scholar] [CrossRef]
- Colmenero, F.; Timón, V. Mechanical anomalies in mercury oxalate and the deformation of the mercury cube coordination environment under pressure. Appl. Phys. A 2021, 127, 395. [Google Scholar] [CrossRef]
- Troullier, N.; Martins, J.L. Efficient Pseudopotentials for Plane-Wave Calculations. Phys. Rev. B 1991, 43, 1993–2006. [Google Scholar] [CrossRef]
- Pfrommer, B.G.; Cote, M.; Louie, S.G.; Cohen, M.L. Relaxation of Crystals with the Quasi-Newton Method. J. Comput. Phys. 1997, 131, 233–240. [Google Scholar] [CrossRef]
- Downs, R.T.; Bartelmehs, K.L.; Gibbs, G.V.; Boisen, M.B. Interactive software for calculating and displaying X-ray or neutron powder diffractometer patterns of crystalline materials. Am. Mineral. 1993, 78, 1104–1107. [Google Scholar]
- Nye, J.F. Physical Properties of Crystals; Clarendon: Oxford, UK, 1976. [Google Scholar]
- Yu, R.; Zhu, J.; Ye, H.Q. Calculations of Single-Crystal Elastic Constants Made Simple. Comput. Phys. Commun. 2010, 181, 671–675. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Martin, R.M. Quantum-mechanical theory of stress and force. Phys. Rev. B 1985, 32, 3780–3791. [Google Scholar] [CrossRef] [PubMed]
- Colmenero, F.; Bonales, L.J.; Cobos, J.; Timón, V. Structural, mechanical and vibrational study of uranyl silicate mineral soddyite by DFT calculations. J. Solid. State Chem. 2017, 253, 249–257. [Google Scholar] [CrossRef]
- Colmenero, F.; Bonales, L.J.; Timón, V.; Cobos, J. Structural, mechanical and Raman spectroscopic characterization of the layered uranyl silicate mineral, uranophane-α, by density functional theory methods. Clay Miner. 2018, 53, 377–392. [Google Scholar] [CrossRef]
- Colmenero, F.; Cobos, J.; Timón, V. Periodic Density Functional Theory Study of the Structure, Raman Spectrum, and Mechanical Properties of Schoepite Mineral. Inorg. Chem. 2018, 57, 4470–4481. [Google Scholar] [CrossRef] [PubMed]
- Colmenero, F.; Fernández, A.M.; Cobos, J.; Timón, V. Becquerelite mineral phase: Crystal structure and thermodynamic and mechanical stability by using periodic DFT. RSC Adv. 2018, 8, 24599–24616. [Google Scholar] [CrossRef]
- Colmenero, F.; Plášil, J.; Sejkora, J. The layered uranyl silicate mineral uranophane-β: Crystal structure, mechanical properties, Raman spectrum and comparison with the α-polymorph. Dalton Trans. 2018, 48, 16722–16736. [Google Scholar] [CrossRef]
- Colmenero, F.; Plášil, J.; Cobos, J.; Sejkora, J.; Timón, V.; Čejka, J.; Bonales, L.J. Crystal structure, hydrogen bonding, mechanical properties and Raman spectrum of the lead uranyl silicate monohydrate mineral kasolite. RSC Adv. 2019, 9, 15323–15334. [Google Scholar] [CrossRef]
- Colmenero, F.; Plášil, J.; Cobos, J.; Sejkora, J.; Timón, V.; Čejka, J.; Fernández, A.M.; Petříček, V. Structural, mechanical, spectroscopic and thermodynamic characterization of the copper-uranyl tetrahydroxide mineral vandenbrandeite. RSC Adv. 2019, 9, 40708–40726. [Google Scholar] [CrossRef]
- Colmenero, F.; Plášil, J.; Němec, I. Uranosphaerite: Crystal structure, hydrogen bonding, mechanics, infrared and Raman spectroscopy and thermodynamics. J. Phys. Chem. Solids 2020, 141, 109400. [Google Scholar] [CrossRef]
- Colmenero, F.; Plášil, J.; Škácha, P. The magnesium uranyl tricarbonate octadecahydrate mineral, bayleyite: Periodic DFT study of its crystal structure, hydrogen bonding, mechanical properties and infrared spectrum. Spectrochim. Acta A 2020, 234, 118216. [Google Scholar] [CrossRef]
- Colmenero, F.; Plášil, J.; Timón, V.; Čejka, J. Full crystal structure, hydrogen bonding and spectroscopic, mechanical and thermodynamic properties of mineral uranopilite. RSC Adv. 2020, 10, 31947–31960. [Google Scholar] [CrossRef]
- Colmenero, F. Anomalous mechanical behavior of the deltic, squaric and croconic cyclic oxocarbon acids. Mater. Res. Express 2019, 6, 045610. [Google Scholar] [CrossRef]
- Colmenero, F. Mechanical properties of anhydrous oxalic acid and oxalic acid dihydrate. Phys. Chem. Chem. Phys. 2019, 21, 2673–2690. [Google Scholar] [CrossRef] [PubMed]
- Colmenero, F. Negative area compressibility in oxalic acid dihydrate. Mater. Lett. 2019, 245, 25–28. [Google Scholar] [CrossRef]
- Colmenero, F. Organic acids under pressure: Elastic properties, negative mechanical phenomena and pressure induced phase transitions in the lactic, maleic, succinic and citric acids. Mater. Adv. 2020, 1, 1399–1426. [Google Scholar] [CrossRef]
- Colmenero, F.; Cobos, J.; Timón, V. Negative linear compressibility in uranyl squarate monohydrate. J. Phys. Cond. Matter. 2019, 31, 175701. [Google Scholar] [CrossRef]
- Colmenero, F.; Timón, V. Extreme negative mechanical phenomena in the zinc and cadmium anhydrous metal oxalates and lead oxalate dihydrate. J. Mater. Sci. 2020, 55, 218–236. [Google Scholar] [CrossRef]
- Colmenero, F. Silver oxalate: Mechanical properties and extreme negative mechanical phenomena. Adv. Theor. Simul. 2019, 2, 1900040. [Google Scholar] [CrossRef]
- Colmenero, F.; Jiang, X.; Li, X.; Li, Y.; Lin, Z. Negative area compressibility in silver oxalate. J. Mater. Sci. 2021, 56, 269–277. [Google Scholar] [CrossRef]
- Baroni, S.; Giannozzi, P.; Testa, A. Elastic Constants of Crystals from Linear-Response Theory. Phys. Rev. Lett. 1987, 59, 2662–2665. [Google Scholar] [CrossRef]
- Wu, X.; Vanderbilt, D.; Hamann, D.R. Systematic treatment of displacements, strains, and electric fields in density-functional perturbation theory. Phys. Rev. B 2005, 72, 035105. [Google Scholar] [CrossRef]
- Wentzcovitch, R.M.; Wu, Z.Q.; Carrier, P. Thermodynamic properties and phase relations in mantle minerals investigated by first principles quasiharmonic theory. Rev. Mineral. Geochem. 2010, 71, 99–128. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Wentzcovitch, R.M. Quasiharmonic thermal elasticity of crystals: An analytical approach. Phys. Rev. B 2011, 83, 184115. [Google Scholar] [CrossRef]
- Parrinello, J.; Rahman, A. Strain fluctuations and elastic constants. J. Chem. Phys. 1982, 76, 2662–2666. [Google Scholar] [CrossRef]
- Ray, J.R. Comput. Ensembles and Computer Simulation Calculation of Response Functions. Phys. Rep. 1988, 8, 109–152. [Google Scholar]
- Wojciechowski, K.W.; Tretiakov, K.V. Quick and accurate estimation of the elastic constants using the minimum image method. Comp. Phys. Commun. 1999, 121–122, 528–530. [Google Scholar] [CrossRef]
- Van Workum, K.V.; De Pablo, J. Local elastic constants in thin films of an fcc cristal. Phys. Rev. E 2003, 67, 011505. [Google Scholar] [CrossRef]
- Voyiatzis, E. Mechanical properties and elastic constants of atomistic systems through the stress-fluctuation formalism. Comput. Phys. Commun. 2013, 184, 27–33. [Google Scholar] [CrossRef]
- Marmier, A.; Lethbridge, Z.A.D.; Walton, R.I.; Smith, C.; Parker, S.C.; Evans, K.E. ElAM: A computer program for the analysis and representation of anisotropic elastic properties. Comput. Phys. Commun. 2010, 181, 2102–2115. [Google Scholar] [CrossRef]
- Lowenstein, W. The distribution of aluminum in the tetrahedra of silicates and aluminates. Am. Mineral. 1954, 39, 91–96. [Google Scholar]
- Davis, M.E.; Hathaway, P.E.; Montes, C. Zeolites and molecular sieves: Not just ordinary catalysts. Zeolites 1980, 9, 436–439. [Google Scholar] [CrossRef]
- Voigt, W. Lehrbuch der Kristallphysik; Teubner: Leipzig, Germany, 1962. [Google Scholar]
- Reuss, A.Z. Berechnung der Fließgrenze von Mischkristallen auf Grund der Plastizitätsbedingung für Einkristalle. Angew. Math. Mech. 1929, 9, 49–58. [Google Scholar] [CrossRef]
- Hill, R. The Elastic Behaviour of a Crystalline Aggregate. Proc. Phys. Soc. Lond. A 1952, 65, 349–354. [Google Scholar] [CrossRef]
- Weck, P.F.; Kim, E.; Buck, E.C. On the mechanical stability of uranyl peroxide hydrates: Implications for nuclear fuel degradation. RSC Adv. 2015, 5, 79090–79097. [Google Scholar] [CrossRef]
- Colmenero Ruiz, F. Theoretical Studies of the Structural, Mechanic and Raman Spectroscopic Properties of Uranyl Containing Minerals. In Minerals; Essa, K.S., Ed.; InTechOpen: London, UK, 2018; Chapter 4; pp. 65–94. [Google Scholar]
- Angel, R.J. Equations of State. Rev. Mineral. Geochem. 2000, 41, 35–60. [Google Scholar] [CrossRef]
- Lacivita, V.; D’Arco, P.; Mustapha, S.; Bernardes, D.F.; Dovesi, R.; Erba, A.; Rérat., M. Ab initio compressibility of metastable low albite: Revealing a lambda-type singularity at pressures of the Earth’s upper mantle. Phys. Chem. Miner. 2020, 47, 45. [Google Scholar] [CrossRef]
- Evans, K.E. Auxetic polymers: A new range of materials. Endeavour 1991, 15, 170–174. [Google Scholar] [CrossRef]
- Colmenero, F.; Sejkora, J.; Plášil, J. Crystal Structure, Infrared Spectrum and Elastic Anomalies in Tuperssuatsiaite. Sci. Rep. 2020, 10, 7510. [Google Scholar] [CrossRef]
- Baughman, R.H.; Fonseca, A.F. Straining to expand entanglements. Nat. Mater. 2015, 15, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Bryukhanov, I.A.; Rybakov, A.A.; Larin, A.V.; Trubnikov, D.N.; Vercauteren, D.P. The role of water in the elastic properties of aluminosilicate zeolites: DFT investigation. J. Mol. Model. 2017, 23, 68. [Google Scholar] [CrossRef] [PubMed]
- Coasne, B.; Haines, J.; Levelut, C.; Cambon, O.; Santoro, M.; Gorelli, F.; Garbarino, G. Enhanced mechanical strength of zeolites by adsorption of guest molecules. Phys. Chem. Chem. Phys. 2011, 13, 20096–20099. [Google Scholar] [CrossRef] [PubMed]
- Mouhat, F.; Bousquet, D.; Boutin, A.; du Bourg, L.B.; Coudert, F.X.; Fuchs, A.H. Softening upon Adsorption in Microporous Materials: A Counterintuitive Mechanical Response. J. Phys. Chem. Lett. 2015, 6, 4265–4269. [Google Scholar] [CrossRef] [PubMed]
- Canepa, P.; Tan, K.; Du, Y.; Lu, H.; Chabal, Y.J.; Thonhauser, T. Structural, elastic, thermal, and electronic responses of small-molecule-loaded metal–organic framework materials. J. Mater. Chem. A 2015, 3, 986–995. [Google Scholar] [CrossRef]
- Hu, Y.; Navrotsky, A. Thermochemical Study of the Relative Stability of Dense and Microporous Aluminophosphate Frameworks. Chem. Mater. 1995, 7, 1816–1823. [Google Scholar] [CrossRef]
- Navrotsky, A.; Petrovic, I.; Hu, Y.; Chen, C.Y.; Davis, M.E. Little energetic limitation to microporous and mesoporous materials. Microporous Mater. 1995, 4, 95–98. [Google Scholar] [CrossRef]
- Boldyreva, E. High-Pressure Polymorphs of Molecular Solids: When Are They Formed, and When Are They Not? Some Examples of the Role of Kinetic Control. Cryst. Growth Des. 2007, 7, 1662–1668. [Google Scholar] [CrossRef]
- Henson, N.J.; Cheetham, A.K.; Gale, J.D. Computational Studies of Aluminum Phosphate Polymorphs. Chem. Mater. 1995, 8, 664–670. [Google Scholar] [CrossRef]
- Fabbiani, M.; Polisi, M.; Fraisse, B.; Arletti, R.; Santoro, M.; Alabarse, F.; Haines, J. An in-situ x-ray diffraction and infrared spectroscopic study of the dehydration of AlPO4-54. Solid State Sci. 2020, 108, 106378. [Google Scholar] [CrossRef]
- Bennett, J.M.; Cohen, J.P.; Flanigen, E.M.; Pluth, J.J.; Smith, J.V. Aluminophosphate molecular sieve AlPO4-11: Partial refinement from powder data using a pulsed neutron source. ACS Symp. Ser. 1983, 218, 109–118. [Google Scholar] [CrossRef]
- Richardson, J.M.; Pluth, J.J.; Smith, J.V. Structure determination and rietveld refinement of aluminophosphate molecular sieve AIPO4-8. Acta Crystallogr. C 1987, 43, 1469–1472. [Google Scholar] [CrossRef]
- Ohnishi, N.; Qiu, S.; Teresaki, O.; Kajitani, T.; Hiraga, K. Hexagonal-orthorhombic phase transformation of AlPO4-5 aluminophosphate molecular sieve. Microporous Mater. 1993, 2, 73–74. [Google Scholar] [CrossRef]
- Ikeda, T.; Miyazawa, K.; Izumi, F.; Huang, Q.; Santoro, A.J. Structural study of the aluminophosphate AlPO4-5 by neutron powder diffraction. J. Phys. Chem. Solids 1999, 60, 1531–1535. [Google Scholar] [CrossRef]
- Polisi, M.; Arletti, R.; Quartieri, S.; Pastero, L.; Giacobbe, C.; Vezzalini, G. Dehydration mechanism of AlPO4-5: A high-resolution synchrotron X-ray powder diffraction study. Microporous Mesoporous Mater. 2018, 261, 137–143. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Huang, Y.J. Ultrafast crystallization of AlPO4-5 molecular sieve in a deep eutectic solvent. Phys. Chem. C 2021, 125, 8876–8889. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Zeng, D.; Yang, J.; Zhang, M.; Ye, C.; Deng, J. Solid-state NMR of silicoaluminophosphate molecular sieves and aluminophosphate materials. J. Phys. Chem. B 2007, 111, 7105–7113. [Google Scholar] [CrossRef]
- Fan, F.; Feng, Z.; Sun, K.; Guo, W.; Guo, Q.; Song, Y.; Li, W.; Li, C. In situ UV Raman spectroscopic study on the synthesis mechanism of AlPO-5. Angew. Chem. Int. Ed. 2009, 121, 8899–8903. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, G.; Wang, L.; Zhang, X. Ionothermal synthesis of phase pure AlPO4-5 using a series of tri-substituted imidazolium bromides. Microporous Mesoporous Mater. 2014, 193, 1–6. [Google Scholar] [CrossRef]
- Chen, B.; Kirby, C.W.; Huang, Y. Investigation of Crystallization of Molecular Sieve AlPO4-5 by the Dry Gel Conversion Method. J. Phys. Chem. C 2009, 113, 15868–15876. [Google Scholar] [CrossRef]
- Sheng, N.; Chu, Y.; Xin, S.; Wang, Q.; Yi, X.; Feng, Z.; Meng, X.; Liu, X.; Deng, F.; Xiao, F.S. Microwave Assisted Green Synthesis, Characterisation of Alanine Templated Aluminophosphate Zeolite and Study of Its Application as Adsorbent. J. Am. Chem. Soc. 2016, 138, 6171–6176. [Google Scholar] [CrossRef]


















| Parameter | ||||||||
|---|---|---|---|---|---|---|---|---|
| VPI-5 | ||||||||
| PBE | 18.5665 | 18.5665 | 8.5330 | 90.0 | 90.0 | 120.0 | 2547.3630 | 1.431 |
| PBE + Disp | 18.5253 | 18.5253 | 8.5234 | 90.0 | 90.0 | 120.0 | 2533.2330 | 1.439 |
| PBEsol | 18.5430 | 18.5430 | 8.5398 | 90.0 | 90.0 | 120.0 | 2542.9495 | 1.433 |
| Exp [4] | 18.6005(6) | 18.6005(6) | 8.3664(4) | 90.0 | 90.0 | 120.0 | 2506.7931 | 1.455 |
| ALPO-8 | ||||||||
| PBE | 33.5899 | 14.7280 | 8.5334 | 90.0 | 90.0 | 90.0 | 4221.5557 | 1.727 |
| PBE + Disp | 33.3954 | 14.6915 | 8.5222 | 90.0 | 90.0 | 90.0 | 4181.1994 | 1.744 |
| PBEsol | 33.5175 | 14.7223 | 8.5398 | 90.0 | 90.0 | 90.0 | 4213.9746 | 1.730 |
| Exp [6] | 33.29(2) | 14.76(2) | 8.257(4) | 90.0 | 90.0 | 90.0 | 4057.1628 | 1.797 |
| ALPO-5 | ||||||||
| PBE | 13.8671 | 13.8671 | 8.5350 | 90.0 | 90.0 | 120.0 | 1421.3670 | 1.710 |
| PBE + Disp | 13.8401 | 13.8401 | 8.5261 | 90.0 | 90.0 | 120.0 | 1414.3565 | 1.718 |
| PBEsol | 13.8645 | 13.8645 | 8.5382 | 90.0 | 90.0 | 120.0 | 1421.3642 | 1.710 |
| Exp [9] | 13.718(1) | 13.718(1) | 8.4526(5) | 90.0 | 90.0 | 120.0 | 1377.5347 | 1.765 |
| ALPO-18 | ||||||||
| PBE | 13.5704 | 12.6773 | 18.4699 | 90.0 | 90.01 | 90.0 | 3177.4870 | 1.529 |
| PBE + Disp | 13.5561 | 12.6613 | 18.4442 | 90.0 | 90.02 | 90.0 | 3165.7084 | 1.535 |
| PBEsol | 13.5788 | 12.6705 | 18.4592 | 90.0 | 90.01 | 90.0 | 3175.9001 | 1.530 |
| Exp [10] | 13.7114(1) | 12.7314(1) | 18.5703(1) | 90.0 | 90.01(1) | 90.0 | 3241.7302 | 1.500 |
| ALPO-18 | ||||||||
| PBE | 9.3276 | 9.4113 | 18.3376 | 88.24 | 91.77 | 88.97 | 1607.9391 | 1.957 |
| PBE + Disp | 9.2582 | 9.3513 | 18.2448 | 87.99 | 91.81 | 89.32 | 1577.6793 | 1.995 |
| PBEsol | 9.2487 | 9.3723 | 18.2507 | 88.66 | 91.93 | 88.54 | 1580.1187 | 1.992 |
| Exp [11] | 9.251 | 9.362 | 18.428 | 90.89 | 96.35 | 90.87 | 1585.7971 | 1.985 |
| ALPO-31 | ||||||||
| PBE | 20.9724 | 20.9724 | 5.0778 | 90.0 | 90.0 | 120.0 | 1934.2242 | 1.884 |
| PBE + Disp | 20.9230 | 20.9230 | 5.0683 | 90.0 | 90.0 | 120.0 | 1921.5046 | 1.897 |
| PBEsol | 20.9635 | 20.9635 | 5.0767 | 90.0 | 90.0 | 120.0 | 1932.1567 | 1.886 |
| Exp [12] | 20.827(1) | 20.827(1) | 5.003(1) | 90.0 | 90.0 | 120.0 | 1879.3798 | 1.940 |
| VPI-5 | ALPO-8 | ALPO-5 | ALPO-18 | ALPO-31 | |
|---|---|---|---|---|---|
| 11 | 85.17 | 82.23 | 124.23 | 105.68 | 109.15 |
| 22 | 85.17 | 102.00 | 124.23 | 98.33 | 109.15 |
| 33 | 158.27 | 187.62 | 192.56 | 105.82 | 138.05 |
| 44 | 19.10 | 23.94 | 26.24 | 13.99 | 30.81 |
| 55 | 19.10 | 25.96 | 26.24 | 18.71 | 30.81 |
| 66 | 21.79 | 23.35 | 28.22 | 28.90 | 23.01 |
| 12 | 41.59 | 53.01 | 67.80 | 68.62 | 63.13 |
| 13 | 43.66 | 48.05 | 59.70 | 57.17 | 61.58 |
| 14 | 0.0 | 0.0 | 0.0 | 0.0 | 5.76 |
| 15 | 0.0 | 0.0 | 0.0 | −2.42 | 0.46 |
| 16 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 23 | 43.66 | 51.17 | 59.70 | 52.13 | 61.58 |
| 24 | 0.0 | 0.0 | 0.0 | 0.0 | −5.76 |
| 25 | 0.0 | 0.0 | 0.0 | −1.73 | −0.46 |
| 26 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 34 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 35 | 0.0 | 0.0 | 0.0 | 3.16 | 0.0 |
| 36 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 45 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 46 | 0.0 | 0.0 | 0.0 | −1.21 | −0.46 |
| 56 | 0.0 | 0.0 | 0.0 | 0.0 | 5.76 |
| Property | VPI-5 | ALPO-8 | ALPO-5 | ALPO-18 | ALPO-31 | |
|---|---|---|---|---|---|---|
| Bulk modulus | 60.49 0.56 | 68.27 0.87 | 88.22 0.60 | 73.56 0.95 | 80.17 1.16 | |
| Shear modulus | 22.67 | 25.56 | 30.24 | 19.34 | 26.41 | |
| Young’s modulus | 60.46 | 68.18 | 81.43 | 53.35 | 71.39 | |
| Poisson’s ratio | 0.33 | 0.33 | 0.35 | 0.38 | 0.35 | |
| Ductility index | 2.66 | 2.67 | 2.92 | 3.80 | 3.04 | |
| Intrinsic ductility index | 0.37 | 0.43 | 0.51 | 1.02 | 0.45 | |
| Hardness index | 0.94 | 1.22 | 1.13 | 0.63 | 0.70 | |
| Universal anisotropy | 0.66 | 0.83 | 0.49 | 0.46 | 0.36 | |
| Bulk modulus (SC) | 60.74 0.81 | 72.60 0.42 | 87.27 1.36 | 73.85 2.92 | 90.14 1.56 | |
| Material | ||||
|---|---|---|---|---|
| VPI-5 ) | 16.46 | 6.95 | 6.95 | 2.56 |
| ALPO-8 ) | 13.77 | 6.93 | 5.04 | 1.81 |
| ALPO-5 | 11.45 | 4.61 | 4.61 | 2.25 |
| ALPO-18 | 13.54 | 3.40 | 5.17 | 4.97 |
| ALPO-31 ) | 11.09 | 4.43 | 4.43 | 2.23 |
| VPI-5 | ALPO-5 | ALPO-18W | |
|---|---|---|---|
| 11 | 69.66 | 120.90 | 92.30 |
| 22 | 68.50 | 127.95 | 103.17 |
| 33 | 79.68 | 193.34 | 103.75 |
| 44 | 18.75 | 26.30 | 25.58 |
| 55 | 19.61 | 26.04 | 24.06 |
| 66 | 19.38 | 25.61 | 25.81 |
| 12 | 31.53 | 67.01 | 37.53 |
| 13 | 14.04 | 60.75 | 42.27 |
| 14 | 0.0 | 0.0 | −2.52 |
| 15 | −0.05 | 0.0 | −5.10 |
| 16 | 0.0 | 0.0 | 7.90 |
| 23 | 12.60 | 62.53 | 41.38 |
| 24 | 0.0 | 0.0 | 6.67 |
| 25 | −0.20 | 0.0 | −0.47 |
| 26 | 0.0 | 0.0 | 3.03 |
| 34 | 0.0 | 0.0 | 0.50 |
| 35 | 0.42 | 0.0 | −3.83 |
| 36 | 0.0 | 0.0 | −1.19 |
| 45 | 0.0 | 0.0 | 1.85 |
| 46 | 0.20 | 0.0 | 0.58 |
| 56 | 0.0 | 0.0 | 1.37 |
| Property | VPI-5 | ALPO-5 | ALPO-18W |
|---|---|---|---|
| 37.06 0.81 | 88.62 0.37 | 58.82 0.94 | |
| 20.98 | 29.58 | 25.81 | |
| 54.24 | 79.87 | 67.56 | |
| 0.26 | 0.35 | 0.31 | |
| 1.77 | 3.00 | 2.28 | |
| 0.24 | 0.51 | 0.18 | |
| 3.10 | 1.02 | 2.11 | |
| 0.29 | 0.50 | 0.24 | |
| 33.85 1.90 | 87.71 0.27 | 58.80 1.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colmenero, F.; Lobato, Á.; Timón, V. Mechanical Characterization of Anhydrous Microporous Aluminophosphate Materials: Tridimensional Incompressibility, Ductility, Isotropy and Negative Linear Compressibility. Solids 2022, 3, 457-499. https://doi.org/10.3390/solids3030032
Colmenero F, Lobato Á, Timón V. Mechanical Characterization of Anhydrous Microporous Aluminophosphate Materials: Tridimensional Incompressibility, Ductility, Isotropy and Negative Linear Compressibility. Solids. 2022; 3(3):457-499. https://doi.org/10.3390/solids3030032
Chicago/Turabian StyleColmenero, Francisco, Álvaro Lobato, and Vicente Timón. 2022. "Mechanical Characterization of Anhydrous Microporous Aluminophosphate Materials: Tridimensional Incompressibility, Ductility, Isotropy and Negative Linear Compressibility" Solids 3, no. 3: 457-499. https://doi.org/10.3390/solids3030032