3.1. Characterization of TiO2 Powder
TiO
2 has been effectively used in cosmetic sunscreens for a long time [
30]. Its safe use as an UV-filter at a maximum concentration of 25% and particle size higher than 100 nm in cosmetic products has already been recognized by the Scientific Committee on Consumer Safety [
31]. It is especially recommended for individuals with a high tendency for skin irritation [
5]. Moreover, TiO
2 is essential for manufacturing sunscreens with a high SPF [
5] and its properties guarantee that formulations are “lighter” and can be spread more regularly on the skin, thus improving the UV protection [
32].
TiO2 appears in two natural forms—anatase and rutile. It is generally assumed that rutile, the most stable polymorph, is also the most effective one due to a larger absorption of UV-R. Additionally, its hydrophobic nature allows a more durable water resistance, which is quite important for sunscreen formulation.
The wettability properties of solid particles determine the water resistance performance of sunscreens. Therefore, oil-wet particles with higher angles in contact with water would be desirable.
Anatase TiO2 presented a contact angle with water of 70.24 ± 5.74°. The contact angle for rutile was higher (105.60 ± 1.17°) due to its higher hydrophobicity. There was a statistically significant difference between these input groups (p < 0.05). Both TiO2 forms presented angles lower than 20° when measured with paraffin.
According to these results, the rutile form would be more suitable for water resistance sunscreens.
According to
Table 2, there were significant differences in particle size which was much higher in rutile form than anatase form with span values of 3821 nm and 595 nm, respectively. In contrary, Teruhisa et al. [
33] reported that average sizes of the anatase and rutile elementary particles (Degussa
®, P-25) determined by transmission electron microscopy were 85 and 25 nm, respectively. Thus, higher Span values here recorded could be associated with an aggregation phenomenon of both forms of TiO
2 in the aqueous medium where measurements were performed. Nevertheless, as this dispersing medium is different from that in the sunscreen formulations produced here, the TiO
2 particles may be dispersed at a finer state in these formulations. In fact, it is prudent to assume this characterization assay could be quite challenging.
3.2. Characterization of TiO2 Sunscreen Formulations
In this assay, the influence of the TiO
2 polymorph on emulsions’ droplet size was evaluated. Theoretically, smaller droplets contribute to obtain a more stable emulsion, as observed in the prototype AC containing anatase TiO
2 and GCO. In this case, a significant percentage of droplets exhibited a size ranging from 0.1 to 0.5 µm differing from the other prototypes (including rutile TiO
2 formulations) about 10 µm (
Figure 1). The peaks around 10 µm for all prototypes except AC could be due to the presence of aggregates, as mentioned before. It can be also observed that a lower droplet size was obtained in the formulation containing P25 and green coffee oil which could be explained by the difference in particle size obtained for each form. Therefore, both TiO
2 form (especially AC vs RC) and oil type (mineral or green coffee oil in each prototype) affected the droplet size distribution of these emulsions. As previously outlined by Marto et al. (2016) [
32], there are several factors that can explain these results, namely the wettability of the solid particles (anatase and rutile) at the oil-water interface also related to their solubility.
- (A)
Dynamic viscosity—Flow curves
The
Figure 2 shows the flow curve of the six emulsions studied. These data show that both anatase and rutile formulations were influenced by the oil type. All formulations could be classified as non-Newtonian fluids with thixotropic hysteresis loops.
The influence of TiO2 on viscosity and thixotropic behavior could be verified comparing the prototypes AC/AM (anatase TiO2) to RC/RM (rutile TiO2). In particular, the anatase form increased the apparent viscosity values at the apex of the curves and rutile increased the thixotropy (especially RM).
The addition of GCO promoted different interactions with rutile and anatase TiO2. When the oil was added to the formulation containing anatase TiO2 (prototype AC), the viscosity decreased and on the contrary, the thixotropy increased. This profile was exactly the opposite regarding the formulation containing rutile TiO2 and GCO (prototype RC).
- (B)
Oscillation measurements
The elastic/storage (G’) and viscous/loss (G”) moduli were obtained from the frequency sweep within the linear viscoelastic region (LVR), previously determined from a stress sweep at a frequency of 1 Hz [
18]. The storage modulus G’ corresponds to the elastic portion of the viscoelastic behavior, which describes the solid-state of the sample representing the stored deformation energy through internal friction between the constituents in a flowing fluid [
17]. The loss modulus G” corresponds to the viscous portion of the viscoelastic behavior, which describes the liquid-state of the sample representing the deformation energy lost through internal friction [
34].
In the frequency sweep assay (
Figure 3), all formulations exhibited G’ values higher than G″ which means that the elastic modulus was predominant and an indicator of high stability of the formulations with strong bonds, both in the presence and absence of GCO.
Considering the formulations containing anatase TiO2 in the presence of the green coffee oil (prototype AC), the G’ values increased and were higher than G″ compared to the mineral oil (prototype AM). The same behavior was observed for formulations containing rutile TiO2 (prototypes RC and RM).
The creep and recovery results are shown in
Figure 4. All formulations were viscoelastic when they were tested in creep mode and exhibited the same general shape. The overall results show that the total compliances (J) of AM, RM and M were higher than AC, RC and C, respectively, implying that GCO formulations possessed a more delicate network than the others. Considering that this behavior is observed in control formulations, the reduction of deformation ability should be attributed to GCO interactions.
The mechanical properties studied by TPA clearly depend on the addition of GCO and TiO
2, as it can be seen on
Table 3 except the cohesiveness that remains similar in all instances.
The presence of GCO increases the value of compressibility, hardness and adhesiveness as shown by the comparison of controls. The inclusion of TiO2 points out an increase of these properties, slightly higher for the rutile form (when comparing data from AM, RM and M formulations). The combination of GCO plus TiO2 enhances this improvement.
Considering that adhesiveness is more related to surface characteristics and depends on a combined effect of adhesive and cohesive forces [
32,
35], it can be considered that the predominance of adhesion forces.
These results also allow the prediction of a higher water resistance for these sunscreen formulations compared to the respective controls.
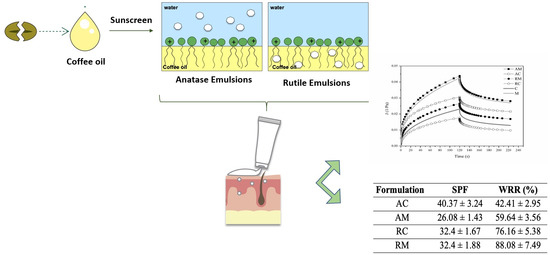
The
Table 4 shows the SPF values and WRR percentage measured for TiO
2 formulations.
Regarding the SPF, the values obtained were superior to 20, and the highest and lowest values corresponded to anatase TiO2 form with GCO (AC) and mineral oil (AM), respectively. Thus, the oil type clearly influenced this result even though the same did not occur in rutile TiO2 formulations (RC and RM) which presented the same SPF. The influence of TiO2 form was better evidenced in absence of coffee oil, where rutile TiO2 (RM) was more effective as an UV attenuator than anatase TiO2 (AM), due to higher refractive index of rutile form. In addition to UV protection conferred by these inorganic filters, it should be also considered the absorbance of UV-B rays by ethylhexyl methoxycinnamate, an organic filter also present in the composition of these prototype formulations.
In addition, another factor that varies the level of protection is the water contact since UV absorbers present in the formulation can leach out or be physically removed by the washing action [
24]. It should be noted that in vivo WRR testing of topical sunscreens is expensive, time consuming and experimentally sticky [
36] and that was why an in vitro method was here used.
Products which claim to be ‘water resistant’ exhibit a gradual decrease in protection over the first immersion but lose extraordinarily little protection over the following immersions. In particular, ‘water resistant’ products should comply with Cosmetics Europe proposed guidelines on water resistance labeling [
24]: the ‘waterproof’ products have a %WRR exceeding 80% after two immersions, while the %WRR of ‘water resistant’ products is more than 50%. This claim can be sustained for all tested formulations except anatase TiO
2 sunscreen with GCO. In fact, rutile TiO
2 sunscreens were more water resistant than anatase ones.
- (A)
In vitro caffeine release
Topical delivery studies were only performed in TiO2 sunscreens containing GCO (AC and RC) since these were the intended formulations besides the improved outcomes previously obtained in other assays.
The release profiles of caffeine from the TiO
2 sunscreens containing GCO (AC and RC) as well as from the control (C) were expressed as percentage of the applied dose and presented in
Figure 5.
The profiles were similar for the three types of formulations pointing a global effect of the basis of emulsion. The release of caffeine starts immediately and increases steadily over 6 h, which was considered the standard interval between doses. The analysis of the percentage released at this point revealed no statistical differences whereas at 24 h, the amount of control released was higher compared to the other two formulations. This result confirms the ability of TiO2 to adsorb caffeine.
The constant release values (Kd) calculated according to Equation (5) are summarized in
Table 5.
Taken the n-value into account, the release can be attributed mainly to a passive diffusion process, as the n values are below 0.5 [
37]. The release rate constants show that the presence of anatase TiO
2 increases this parameter due to its hydrophilic nature. In contrast, rutile TiO
2 can retain the active compound (i.e., caffeine) and decreases the release rate. Considering that the pharmaceutical form of these formulations is an emulsion, this finding implies that this release is obtained by diffusion of the active compound from the aqueous external phase. Thus, the oily internal phase has probably a depot effect, as caffeine is in equilibrium between the two phases. As a sunscreen product, this result is desired since the active is not rapidly released, reducing the possibility of its permeation through the deeper skin layers, as further confirmed.
- (B)
In vitro caffeine permeability
The TiO
2 particles are not expected to diffuse through the intact skin due to their size (>100 nm). On the contrary, the main active component of the GCO, caffeine, has a well-known ability to diffuse through skin to the deeper layers [
38], where it can produce its antioxidant and protective activity. To check if the active compound would reach the target tissue, a permeability study using Franz diffusion cells and porcine skin (a widely accepted surrogate for human skin) was conducted. In this case, the GCO (only the oil) and a caffeine solution were also used as controls to compare the permeability profile.
The results are shown in
Figure 6. As the permeability of the oil was 30-fold higher than the rest of the formulations, the emulsion creams and the solutions are separately represented.
The parameters calculated after applying the linear regression of the values correspondent to the steady state of each curve are summarized in
Table 6.
The lag time (
tL) varied between 0.1 to 3.4 h. As the linear regression fitting was inaccurate regarding this parameter, it can be concluded from the obtained results that the steady state was reached quite soon and faster in the emulsion sunscreens than the caffeine solution, even though slower than the green coffee oil (GCO). The occlusive effect of the oil, the higher caffeine content, and the presence of an oily medium, that could somehow interact with the lipids of the stratum corneum lipid matrix could be responsible for this effect. In fact, several fatty acids present in oils (including this one) have been reported as permeability enhancers, such as oleic acid [
37].
The permeability coefficient (
Kp) is usually estimated at the steady state, when the drug is concentrated near the saturation range dividing the flux by the drug concentration in the donor compartment [
39]. Even though the flux (
J) is the parameter that directly shows the access of drug to the lower compartment, the
Kp allows a direct comparison between formulations, as this value considers the possible differences between the applied doses.
Considering the
Kp values, the most efficient formulation was the control C, followed by the caffeine solution, RC, AC and then, the GCO, without statistical significance. It might be possible that some ingredients from the emulsion formulations (e.g., propylene glycol or even the surfactants) could act as permeability enhancers of caffeine through the skin, overlapping the expected retarding effect due to the increased viscosity of emulsion prototypes compared to the solution [
37]. In addition, no significant differences in skin permeability were observed between rutile and anatase TiO
2 sunscreen formulations. Up to our knowledge, no data concerning the influence of TiO
2 on the skin permeability has been published to state that rutile or anatase forms could influence the permeability of caffeine.
As it can be observed from these results, GCO showed the highest flux (J). This fact was expected considering that it was the most occlusive formulation (only oil) besides containing the highest concentration of caffeine. However, when the fluxes were normalized with the active concentration in the donor samples, the permeability of caffeine was more efficient, as the permeability coefficient of caffeine solution to the aqueous medium was practically twice than the GCO one (p < 0.05). This effect could be due to the higher bioavailability of the drug when dissolved in an aqueous solution as well as to the depot effect of the GCO in caffeine permeability. At least, no significant differences between the flux values were obtained when comparing both TiO2 sunscreen formulations among each other and the other caffeine controls.