Metal Ions, Element Speciation Forms Retained on Wet Chitin: Quantitative Aspects of Adsorption and Implications for Biomonitoring and Environmental Technology
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
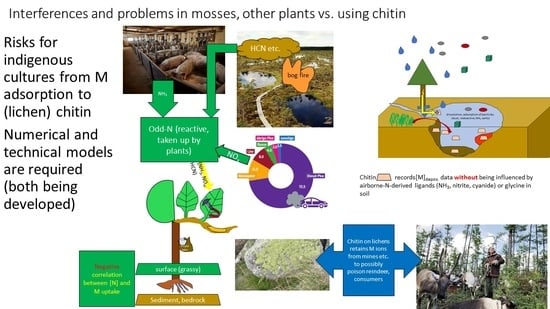
4. Applications to Environmental Safeguarding
5. Conclusions
6. Outlook
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinto, P.X.; Al-Abed, S.R.; Reisman, D.J. Biosorption of heavy metals from mining influenced water onto chitin products. Chem. Eng. J. 2011, 166, 1002–1009. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Bhatnagar, A.; Bikiaris, D.N.; Kyzas, G.Z. Chitin Adsorbents for Toxic Metals: A Review. Int. J. Mol. Sci. 2017, 18, 114–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muzzarelli, R.A.A. Uptake of nitrosyl 106-ruthenium on chitin and chitosan from waste solutions and polluted sea-water. Water Res. 1970, 4, 451–455. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Rocchetti, R.; Marangio, G. Separation of zirconium, niobium, cerium and ruthenium on chitin and chitosan columns for the determination of cesium in nuclear fuel solutions. J. Radioanal. Chem. 1972, 10, 17–25. [Google Scholar] [CrossRef]
- Fränzle, S.; Erler, M.; Blind, F.; Ariuntsetseg, L.; Narangarvuu, D. Chitin adsorption in environmental monitoring: Not an alternative to moss monitoring but a method providing (lots of) bonus information. J. Sci. Arts Univ. Valahia. 2019, 19, 659–674. [Google Scholar]
- Blind, F.; Fränzle, S. Chitin as a sorbent superior to other biopolymers: Features and applications in environmental research, energy conversion, and understanding evolution of animals. Polysaccharides 2021, 2, 773–794. [Google Scholar] [CrossRef]
- Retschke, D. Orientierende Untersuchungen zur Adsorption von Schwermetallen (Nickel) unter dem Einfluss Ausgewählter Komplexliganden Sowie in Arealen Potenzieller und Manifester Methanogenese. Master’s Thesis, International School Zittau (IHI), TUD University of Technology Dresden, Zittau, Germany, 2017. [Google Scholar]
- Fränzle, S.; Blind, F. Polysaccharide (interface) films as catalytic sites, photoreactors producing nutrients and repellants, and electrochemical signals: Kinds and ecological significance of processes in biofilms, chitin in water. Polysaccharides 2022. in print. [Google Scholar]
- Fränzle, S. Chitin on organism covers, controlling transport and measurement of airborne and aquatic pollutants. In Proceedings of the Abstracts IXth BIOMAP Conference, Naples, Napoli, Italy, 3–5 October 2022. [Google Scholar]
- Strasdeit, H. The first cadmium-specific enzyme. Angew. Chem. Engl. Ed. 2001, 40, 707–709. [Google Scholar] [CrossRef]
- L’Vov, N.P.; Nosikov, A.N.; Antipov, A.N. Tungsten-containing enzymes. Biochemistry 2002, 67, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Irgolic, K.J. Arsenic in the Environment. In Xavier AV: Frontiers in Bioinorganic Chemistry; VCH: Weinheim, Germany, 1986; pp. 399–408. [Google Scholar]
- Fränzle, S. Zooplankton, crayfishes and grafted chitin moving in water: Active transport of M ions, complexes and activation of biochemical pathways in sediments due to actively migrating animals and role of chitinases. Polysaccharides 2023, 4. submitted invited paper. [Google Scholar]
- Bauer, A. Orientierende Untersuchungen zur Bindung von Metallionen an Chitin und zur Davon Abhängigen Eignung von Arthropoden zur Bestimmung von Metallionenkonzentrationen in der Umwelt. Master’s Thesis, International School Zittau (IHI), TUD University of Technology Dresden, Zittau, Germany, 2014. [Google Scholar]
- Zones, S.I.; Palmer, M.R.; Palmer, J.G.; Doemeny, J.M.; Schrauzer, G.N. Hydrogen evolving systems. 3. Further observations on the reduction of molecular nitrogen and of other substrates in the vanadium(II) hydroxide-magnesium hydroxide system. J. Am. Chem. Soc. 1978, 100, 2113–2121. [Google Scholar] [CrossRef]
- Kokubo, Y.; Yamamoto, C.; Tsuzuki, K.; Nagai, T.; Katayama, A.; Ohta, T.; Ogura, T.; Wasada-Tsutsui, Y.; Kajita, Y.; Kugimiya, S.; et al. Dinitrogen Fixation by Vanadium Complexes with a Triamidoamine Ligand. Inorg. Chem. 2018, 57, 11884–11894. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, B.; Fränzle, S. (IHI Zittau): Metallgehalte in Wasser und Eisenaquoxidgelen der Quellbecken von Širny Pramen (Zittauer Gebirge, Okres Liberec, Tschechien). Zittau, Germany, 2009, unpublished report.
- Seelmann-Eggebert, W.; Pfennig, G.; Münzel, H.; KLewe-Nebenius, H. Karlsruher Nuklidkarte, 6th ed.; Kernforschungszentrum: Karlsruhe, Germany, 1981; own print KFZ Karlsruhe. [Google Scholar]
- Köllner, K.E.; Carstens, D.; Keller, E.; Vazquez, F.; Schubert, C.J.; Zeyer, J.; Bürgmann, H. Bacterial chitin hydrolysis in two lakes with contrasting trophic statuses. Appl. Environ. Microbiol. 2012, 78, 695–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budelmann, P. Verbreitung der Flusskrebse (Decapoda) in der Südlichen Oberlausitz und Die Eignung des Invasiven Kamberkrebses (Orconectes limosus) für Chitin-Basiertes Monitoring von Schwermetallen in limnischen Ökosystemen. Master’s Thesis, International School Zittau (IHI), TUD University of Technology Dresden, Zittau, Germany, 2021. [Google Scholar]
- Blind, F. Orientierende Untersuchungen zur Platinmetall Freien Aktivierung von CH-Bindungen für Europium Basierte Brennstoffzellenanwendungen. Master’s Thesis, International School Zittau (IHI), TUD University of Technology Dresden, Zittau, Germany, 2018. [Google Scholar]
- Schedlbauer, O.F.; Heumann, K.G. Biomethylation of thallium by bacteria and first determination of biogenic dimethylthallium in the ocean. Appl. Organomet. Chem. 2000, 14, 330–340. [Google Scholar] [CrossRef]
- Vold, I.M.; Christensen, B.E. Periodate oxidation of chitosans with different chemical compositions. Carbohydr. Res. 2005, 340, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Franich, R.; Singh, A.; Gallagher, S.; Kroese, H.; Hill, S. Comment on the structure of osmium tetroxide-chitosan complex. Comments Inorg. Chem. 2011, 32, 39–52. [Google Scholar] [CrossRef]
- Huang, K.; Liu, H.-W.; Dou, X.; Huang, M.-Y.; Jiang, Y.-Y. Silica-supported chitosan–osmium tetroxide complex catalyzed vicinal hydroxylation of olefins using hexacyanoferrate(III) ion as a cooxidant. Polym. Adv. Technol. 2003, 14, 365–372. [Google Scholar] [CrossRef]


| Element | Level in Water [µg/L; nMol/L] | Level on Chitin [µg/L; nMol/L] | Log PF | Level on Chitin in Sediment [µg/L; nMol/L] | Log Ratio PF′ |
|---|---|---|---|---|---|
| M3+ | |||||
| Al | 21.82 | 16.25 | −0.128 | 109.68 | 0.829 |
| Cr | 2.78 | 42.72 | 1.187 | 62.13 | 0.163 |
| Bi | 0.18 | 0.04 | −0.653 | 0.04 | 0 |
| M2+ | |||||
| Ca | 89,200 | 70 | −3.1053 | 60 | −0.067 |
| Sr | 255.7 | 0.78 | −2.5156 | 0.32 | −0.387 |
| Ba | 104.09 | 0.99 | −2.022 | 0.36 | −0.439 |
| Mn | 645.57 | 1.62 | −2.600 | 1.02 | −0.201 |
| Co | 3.04 | 0.18 | −1.228 | 0.14 | −0.109 |
| Ni | 8.23 | 2.57 | −0.5055 | 3.77 | +0.166 |
| Cu | 2.27 | 1.18 | −0.284 | 1.03 | −0.059 |
| Zn | 10.43 | 12.51 | +0.079 | 11.57 | −0.034 |
| Cd | 0.01 | 0.11 | 1.041 | 0.09 | −0.087 |
| Pb | 0.03 | 0.16 | 0.727 | 0.14 | −0.058 |
| Other or unknown oxidation states | |||||
| V | 0.69 | 5.11 | 0.870 | 8.42 | 0.217 |
| Mo | 0.08 | 0.12 | 0.176 | 0.10 | −0.079 |
| U | 0.02 | 0 | - | 0 | - |
| Ag | Not detected (<0.005) | 0.18 | 0.30 | 0.222 |
| Scenario, Conditions, Examples of Metal Ions | Oxidation State, Formula, Range of Corresponding Parameters | Picture | Results |
|---|---|---|---|
| water→chitin | |||
| In-water M transport on chitin; sufficiently short desorption timescale (24 h or less), PFaq ≥ 1 alkaline earth, Mn | M2+ k = −0.0831 a − 1.166, that is, k = −1.34…−0.86 (Cu, Pb) → PF = 2.8…1.8 |  | M release from chitin (lower water column) into open water does escape M biomagnification in zooplankton predators but enables M-dependent biochemical activities at sediment interface and above; Mg and Ca desorb so fast that they are not found in prewashed shrimp chitin, whereas some Sr, Ba, and particularly REEs are retained |
| Same, ferric ditch (FOBs) near Lake Olbersdorf | M2+ k = −0.3914 a − 0.697 | M = Sr, Ba, Co, Ni, and Cu | |
| REEs ≠ Y | M3+ K = −0.0113 a − 0.894, that is, k = − 1.04 (Al, Ti(III))…−0.81 (Ga) → PF ≈ 2.05…1.69 | same | Methanol oxidation by bacteria operating in oxidizing top sediment layers or aq. slurries |
| Same, ferric ditch (FOBs) near Lake Olbersdorf | M3+ K = −0.0875 a + 0.960 | ||
| Higher oxid. State, e.g., VO2+ for which a ≈ +3 | Possibly same | No detailed statement is possible as yet, e.g., N2 assimilation or phenol arene ring halogenations using V | |
| Long τdesorb (>>24 h); heavy 3d ions (Co…Zn), and Cd, Pb, Y | Transfer within water takes place only by predation (M biomagnification in zooplankton predators [fishes, dragonfly nymphs, certain birds]), otherwise deposition to sediment | ||
| sediment→chitin | |||
| M transfer to sediment upon single or repeated contact (leg tips, partly benthic or digging chitin-clad organisms), (or) action of chitinases in sediment after chitin was discarded (molting, ant wings, …) or deposited from dead organisms; effective if PFchitin,sedim./chitin,water > 1 | M2+ for grafted chitin: K = 0.2394 a − 1.761, that is, k = −2.65 (Cu)…−1.30; PF ≈ 22.85…2.6 |  | Includes leg tips (pronounced transport of Mn) Range: grafted chitin: PF′ = 8.2 (Cu, Pb)…1.45 (Ba) Leg tips: PF = 0.65 (Cu, Pb)…1.45Non-equilibrium increasing transfer occurs when there is associated biological activity (Ni) |
| Same, ferric ditch (FOBs) near Lake Olbersdorf | M2+: k = −0.4799 a − 1.946 for grafted chitin | M = Sr, Ba, Co, Ni, Cu; including Mn: M2+: k = −0.4954 a − 2.042 for grafted chitin | |
| M3+ for grafted chitin: K = −0.0422 a − 0.778 that is, k = −1.30 (Al)…−0.47 (Ga): PF ≈ 2.61…1.32 | same | Range grafted chitin: PF′ = 1.27 (Al)…0.78 (Ga) Leg tips: PF ≈ 0.9 for all M(III) ionsnon-equilibrium increasing transfer to chitin in sediment occurs when there is associated biological activity (V, La, other LREEs) | |
| Same, ferric ditch (FOBs) near Lake Olbersdorf | M3+ for grafted chitin: K = −0.0431 a + 1.238 |
| Oxidation State | Formula Water Chitin in Normal Conditions | Formula Water Chitin in Ferric Gel | Difference | Formula Sediment Chitin in Normal Conditions | Formula Sediment Chitin in Ferric Gel | Difference | Remarks, Crossover Points |
|---|---|---|---|---|---|---|---|
| +II | k = −0.0831 a − 1.166 | k = −0.3914 a − 0.697 | Δk = 0.469 − 0.3083 a | k = 0.2394 a − 1.761 | k = −0.4954 a − 2.042 | Δk = −0.281 − 0.7348 a | K gets smaller when a > 1.52 in water (<−1.29; PF ≥ 2.7) and a > −0.38 in sediment (k < −1.85; PF > 5.64) |
| +III | k = −0.0113 a − 0.894 | k = −0.0875 a + 0.960 | Δk = 1.854 − 0.0762 a | k = −0.0422 a − 0.778 | k = −0.0431 a + 1.238 | Δk = 2.016 − 0.0009 a | k cannot become smaller by the presence of MaFexSyOz *n H2O next to either water or sediment |
| Element/Metal | Drinking Water Limit [µg/L] (EPA, EU Regulations) | Drinking Water Limit [nMol/L] | a | K; PF in Water | K; PF in Sediment | PF′, Possible Deviations |
|---|---|---|---|---|---|---|
| Zn | 5000 | 76,500 | −1.42 | −1.28; 2.66 | −1.42; 3.14 | 1.18; deviation is always expected due to key biochemical roles of Zn |
| Cd | 0.5 | 4 | −0.89 | −1.24; 2.54 | −1.55; 3.70 | 1.46; deviations possibly occur in mangrove |
| Pb | 10 | 50 | −3.5 | −1.46; 3.28 | −0.92; 1.84 | 0.56; deviations possibly associated with sulfate reduction? |
| U | 2 (EPA 30) | 8.5 (EPA 130) | not yet determined | Cannot be calculated because oxid. state differs | Cannot be calculated because oxid. state differs | - |
| Ni | 20 | 300 | −2.50 | −0.87; 1.75 | −0.88; 1.78 | 1.02 |
| Cu | 1300 | 20,000 | −3.73 | −1.48; 3.36 | −2.65; 22.74 | 6.76 |
| Mn | 300 | 5500 | −0.09 | −1.17; 2.36 | −1.78; 5.09 | 2.16; likely responds to redox gradient in top sediment layers→PF′increases to ≤50! |
| Cr | 100 | 1900 | −2.60 (assuming Cr3+) | −0.92; 1.84 | −0.89; 1.78 | (0.97); total Cr limit because there is a rapid change among oxidation states, e.g., catalyzed by ambient MnO2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fränzle, S. Metal Ions, Element Speciation Forms Retained on Wet Chitin: Quantitative Aspects of Adsorption and Implications for Biomonitoring and Environmental Technology. Pollutants 2023, 3, 337-350. https://doi.org/10.3390/pollutants3030023
Fränzle S. Metal Ions, Element Speciation Forms Retained on Wet Chitin: Quantitative Aspects of Adsorption and Implications for Biomonitoring and Environmental Technology. Pollutants. 2023; 3(3):337-350. https://doi.org/10.3390/pollutants3030023
Chicago/Turabian StyleFränzle, Stefan. 2023. "Metal Ions, Element Speciation Forms Retained on Wet Chitin: Quantitative Aspects of Adsorption and Implications for Biomonitoring and Environmental Technology" Pollutants 3, no. 3: 337-350. https://doi.org/10.3390/pollutants3030023