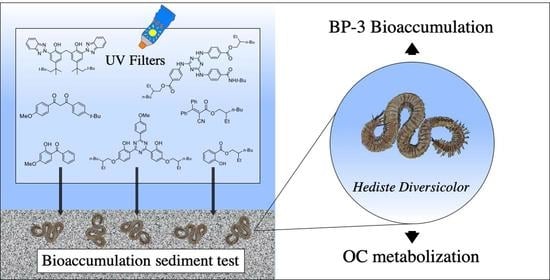
Transfer of 7 Organic UV Filters from Sediment to the Ragworm Hediste diversicolor: Bioaccumulation of Benzophenone-3 and Further Proof of Octocrylene Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Artificial Sediment Preparation and Spiking
2.3. Supply and Acclimatization of Worms
2.4. Bioaccumulation Test in a Spiked Water–Sediment System
2.5. Extraction and Quantification of UV Filters
2.6. Lipid Quantification
2.7. UHPLC-HRMS Profiling and Research of OC Metabolites
2.8. Statistical Analyses
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lozano, C.; Givens, J.; Stien, D.; Matallana-Surget, S.; Lebaron, P. Bioaccumulation and toxicological effects of UV-filters on marine species. In Sunscreens in Coastal Ecosystems: Occurrence, Behavior, Effect and Risk; The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2020; Volume 94, pp. 85–130. [Google Scholar]
- Huang, Y.; Law, J.C.; Lam, T.K.; Leung, K.S. Risks of organic UV filters: A review of environmental and human health concern studies. Sci. Total Environ. 2021, 755, 142486. [Google Scholar] [CrossRef]
- Ramos, S.; Homem, V.; Alves, A.; Santos, L. Advances in analytical methods and occurrence of organic UV-filters in the environment—A review. Sci. Total Environ. 2015, 526, 278–311. [Google Scholar] [CrossRef] [Green Version]
- Fagervold, S.K.; Rodrigues, A.M.S.; Rohée, C.; Roe, C.; Bourrain, M.; Stien, D.; Lebaron, P. Occurrence and environmental distribution of 5 UV filters during the summer season in different water bodies. Water Air Soil Pollut. 2019, 230, 172. [Google Scholar] [CrossRef]
- Sang, Z.; Leung, K.S.-Y. Environmental occurrence and ecological risk assessment of organic UV filters in marine organisms from Hong-Kong coastal waters. Sci. Total Environ. 2016, 566, 489–498. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Hain, E.; Timm, A.; Tarnowski, M.; Blaney, L. Occurrence of antibiotics, estrogenic hormones, and UV-filters in water, sediment, and oyster tissue from the Chesapeake Bay. Sci. Total Environ. 2019, 650, 3101–3109. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Fan, Y.; Jin, J.; Xiong, S.; Liu, J.; Tang, C. Bioaccumulation and biomagnification of ultraviolet absorbents in marine wildlife of the Pearl River Estuarine, South China Sea. Environ. Pollut. 2017, 225, 55–65. [Google Scholar] [CrossRef]
- Cunha, S.C.; Trabalón, L.; Jacobs, S.; Castro, M.; Fernandez-Tejedor, M.; Granby, K.; Verbeke, W.; Kwadijk, C.; Ferrari, F.; Robbens, J.; et al. UV-filters and musk fragrances in seafood commercialized in Europe Union: Occurrence, risk and exposure assessment. Environ. Res. 2018, 161, 399–408. [Google Scholar] [CrossRef]
- Tsui, M.M.P.; Lam, J.C.W.; Ng, T.Y.; Ang, P.O.; Murphy, M.B.; Lam, P.K.S. Occurrence, distribution, and fate of organic UV filters in coral communities. Environ. Sci. Technol. 2017, 5, 4182–4190. [Google Scholar] [CrossRef]
- Mitchelmore, C.L.; He, K.; Gonsior, M.; Hain, E.; Heyes, A.; Clark, C.; Younger, R.; Schmitt-Kopplin, P.; Feerick, A.; Conway, A.; et al. Occurrence and distribution of UV-filters and other anthropogenic contaminants in coastal surface water, sediment, and coral tissue from Hawaii. Sci. Total Environ. 2019, 670, 398–410. [Google Scholar] [CrossRef]
- Langford, K.H.; Reid, M.J.; Fjeld, E.; Øxnevad, S.; Thomas, K.V. Environmental occurrence and risk of organic UV filters and stabilizers in multiple matrices in Norway. Environ. Int. 2015, 80, 1–7. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Alonso, M.B.; Bertozzi, C.P.; Marigo, J.; Barbosa, L.; Cremer, M.; Secchi, E.R.; Domit, C.; Azevedo, A.; Lailson-Brito, J., Jr.; et al. First Determination of UV Filters in Marine Mammals. Octocrylene Levels in Franciscana Dolphins. Environ. Sci. Technol. 2013, 47, 5619–5625. [Google Scholar] [CrossRef] [PubMed]
- Molins-Delgado, D.; Máñez, M.; Andreu, A.; Hiraldo, F.; Eljarrat, E.; Barceló, D.; Díaz-Cruz, M.S. A Potential New Threat to Wild Life: Presence of UV Filters in Bird Eggs from a Preserved Area. Environ. Sci. Technol. 2017, 51, 10983–10990. [Google Scholar] [CrossRef] [PubMed]
- Davey, J.T. The architecture of the burrow of Nereis diversicolor and its quantification in relation to sediment-water exchange. J. Exp. Mar. Biol. Ecol. 1994, 179, 115–129. [Google Scholar] [CrossRef]
- Scaps, P. A review of the biology, ecology and potential use of the common ragworm Hediste diversicolor (O. F. Müller) (Annelida: Polychaeta). Hydrobiologia 2002, 470, 203–218. [Google Scholar] [CrossRef]
- Driscoll, S.K.; McElroy, A.E. Bioaccumulation and metabolism of benzo(a)pyrene in three species of polychaete worms. Environ. Toxicol. Chem. 1996, 15, 1401–1410. [Google Scholar] [CrossRef]
- Cong, Y.; Banta, G.T.; Selck, H.; Berhanu, D.; Valsami-Jones, E.; Forbes, V.E. Toxicity and bioaccumulation of sediment-associated silver nanoparticles in the estuarine polychaete, Nereis (Hediste) diversicolor. Aquat. Toxicol. 2014, 156, 106–115. [Google Scholar] [CrossRef]
- Picone, M.; Delaney, E.; Tagliapietra, D.; Guarneri, I.; Ghirardini, A.V. Bioaccumulation of polychlorinated dibenzo-p-Dioxins (PCDDs) and dibenzofurans (PVDFs) in Hediste diversicolor (polychaeta: Nereididae). Front. Ecol. Evol. 2020, 8, 235. [Google Scholar] [CrossRef]
- Ruus, A.; Schaanning, M.; Øxnevad, S.; Hylland, K. Experimental results on bioaccumulation of metals and organic contaminants from marine sediments. Aquat. Toxicol. 2005, 72, 273–292. [Google Scholar] [CrossRef]
- Le Bihanic, F.; Perrichon, P.; Landi, L.; Clérandeau, C.; Le Menach, K.; Budzinski, H.; Cousin, X.; Cachot, J. Development of a reference artificial sediment for chemical testing adapted to the MELA sediment contact assay. Environ. Sci. Pollut. 2014, 21, 13689–13702. [Google Scholar] [CrossRef]
- Test No. 225: Sediment-Water Lumbriculus Toxicity Test Using Spiked Sediment; OECD Guidelines for the Testing of Chemicals, Section 2; Éditions OCDE: Paris, France, 2007.
- Test No. 315: Bioaccumulation in Sediment-Dwelling Benthic Oligochaetes; OECD Guidelines for the Testing of Chemicals, Section 3; Éditions OCDE: Paris, France, 2008.
- Rodrigues, A.M.S.; Lebaron, P.; Downs, C.A.; Stien, D. Optimization method for titrating sunscreen organic ultraviolet filters in coastal sands. J. Sep. Sci. 2021, 44, 3338–3347. [Google Scholar] [CrossRef]
- Barnes, H.; Blackstock, J. Estimation of lipids in marine animals and tissues: Detailed investigation of the sulphophosphovanillin method for ‘total’ lipids. J. Exp. Mar. Biol. Ecol. 1973, 12, 103–118. [Google Scholar] [CrossRef]
- Inouye, L.S.; Lotufo, G.R. Comparison of macro-gravimetric and micro-colorimetric lipid determination methods. Talanta 2006, 70, 584–587. [Google Scholar] [CrossRef]
- Stien, D.; Clergeaud, F.; Rodrigues, A.M.S.; Lebaron, K.; Pillot, R.; Romans, P.; Fagervold, S.; Lebaron, P. Metabolomics reveal that octocrylene accumulates in Pocillopora damicornis tissues as fatty acid conjugates and triggers coral cell mitochondrial dysfunction. Anal. Chem. 2019, 91, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Blüthgen, N.; Zucchi, S.; Fent, K. Effects of the UV-filter benzophenone-3 (oxybenzone) at low concentrations in zebrafish (Danio rerio). Toxicol. Appl. Pharmacol. 2012, 263, 184–194. [Google Scholar] [CrossRef]
- Yang, H.; Lu, G.; Yan, Z.; Liu, J.; Dong, H.; Bao, X.; Zhang, X.; Sun, Y. Residues, bioaccumulation, and trophic transfer of pharmaceuticals and personal care products in highly urbanized rivers affected by water diversion. J. Hazard. Mater. 2020, 391, 122245. [Google Scholar] [CrossRef]
- Schreurs, R.; Lanser, P.; Seinen, W.; van der Burg, B. Estrogenic activity of UV-filters determined by an in vitro reporter gene assay and an in vivo transgenic Zebrafish assay. Arch. Toxicol. 2002, 76, 257–261. [Google Scholar] [CrossRef]
- Schneider, S.L.; Lim, H.W. Review of environmental effects of oxybenzone and other sunscreen active ingredients. J. Am. Acad. Dermatol. 2019, 80, 266–271. [Google Scholar] [CrossRef]
- Tao, J.; Bai, C.; Chen, Y.; Zhou, H.; Liu, Y.; Shi, Q.; Pan, W.; Dong, H.; Li, L.; Xu, H.; et al. Environmental relevant concentrations of benzophenone-3 induced developmental neurotoxicity in zebrafish. Sci. Total Environ. 2020, 721, 137686. [Google Scholar] [CrossRef]
- Downs, C.A.; Kramarsky-Winter, E.; Segal, R.; Fauth, J.; Knutson, S.; Bronstein, O.; Ciner, F.R.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the U.S. Virgin Islands. Arch. Environ. Contam. 2016, 70, 265–288. [Google Scholar] [CrossRef]
- Bury, D.; Belov, V.; Yulin, Q.; Hayen, H.; Volmer, D.A.; Brüning, T.; Koch, H.M. Determination of urinary metabolites of the emerging UV filter octocrylene by online-SPE-LC-MS/MS. Anal. Chem. 2018, 90, 944–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bury, D.; Modick-Biermann, H.; Leibold, E.; Brüning, T.; Koch, H.M. Urinary metabolites of the UV filter octocrylene in humans as biomarkers of exposure. Arch. Toxicol. 2019, 93, 1227–1238. [Google Scholar] [CrossRef] [PubMed]


| Abbr. a | COSING Names (Alternative Names) | CAS Number | logP b | Formula | MW c (g.mol−1) |
|---|---|---|---|---|---|
| BEMT | bis-Ethylhexyloxyphenol methoxyphenyl triazine (Bemotrizinol) | 187393-00-6 | 10.627 | C38H49N3O5 | 627.8 |
| BM | Butyl methoxydibenzoylmethane (Avobenzone) | 70356-09-1 | 5.499 | C20H22O3 | 310.4 |
| BP3 | Benzophenone-3 (Oxybenzone) | 131-57-7 | 3.514 | C14H12O3 | 228.8 |
| DBT | Diethylhexyl butamido triazone (Iscotrizinol) | 154702-15-5 | 12.004 | C44H59N7O5 | 766.0 |
| ES | 2-Ethylhexyl salicylate (Octyl salicylate, Octisalate) | 118-60-5 | 5.335 | C15H22O3 | 250.3 |
| MBBT | Methylene bis-benzotriazolyl tetramethylbutylphenol (Bisoctrizole) | 103597-45-1 | 15.451 | C41H50N6O2 | 658.9 |
| OC | Octocrylene | 6197-30-4 | 7.083 | C24H27NO2 | 361.5 |
| Csed/D0 a | Csed/D28 a | Lipid Cont. b | Cworms/d28 c | BSAF | |
|---|---|---|---|---|---|
| BEMT | 6.7 ± 1.1 | 6.7 ± 1.3 | 130.9 ± 11.2 | 2.5 ± 0.8 | 0.2 ± 0.0 |
| BM | 2.9 ± 0.5 | 2.9 ± 0.7 | 81.8 ± 8.9 | 0.4 ± 0.1 | 0.1 ± 0.0 |
| BP3 | 5.0 ± 1.2 | 2.5 ± 0.5 | 62.7 ± 8.6 | 33.9 ± 10.0 | 12.4 ± 4.6 |
| DBT | 7.0 ± 3.3 | 6.4 ± 1.4 | 104.7 ± 8.8 | 4.5 ± 0.8 | 0.3 ± 0.0 |
| ES | 4.0 ± 1.8 | 3.6 ± 2.2 | 88.4 ± 3.8 | 1.6 ± 0.3 | 0.3 ± 0.1 |
| MBBT | 6.4 ± 1.6 | 5.5 ± 2.9 | 75.7 ± 1.8 | 1.3 ± 1.1 | 0.1 ± 0.0 |
| OC | 6.1 ± 1.6 | 7.2 ± 1.0 | 64.7 ± 3.5 | 2.2 ± 0.3 | 0.2 ± 0.0 |
| Cmpd. # | –O2CR | tR (min) | Exp. m/z a | Rel. Area b |
|---|---|---|---|---|
| 1 | C16:0 | 15.44 | 638.4177 | 543 |
| 2 | C18:0 | 16.46 | 666.4500 | 553 |
| 3 | C18:1 | 15.52 | 664.4338 | 713 |
| 4 | C18:1 | 15.67 | 664.4330 | 384 |
| 5 | C20:1 | 16.51 | 692.4647 | 1767 |
| 6 | C20:2 | 15.67 | 690.4496 | 676 |
| 7 | C20:2 | 15.77 | 690.4495 | 749 |
| 8 | C20:3 | 14.99 | 688.4339 | 120 |
| 9 | C20:5 | 13.71 | 684.4025 | 47 |
| 10 | C22:2 | 16.63 | 718.4806 | 263 |
| 11 | C22:6 | 14.05 | 710.4181 | 54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clergeaud, F.; Fagervold, S.K.; Rodrigues, A.M.S.; Thorel, E.; Stien, D.; Lebaron, P. Transfer of 7 Organic UV Filters from Sediment to the Ragworm Hediste diversicolor: Bioaccumulation of Benzophenone-3 and Further Proof of Octocrylene Metabolism. Pollutants 2022, 2, 23-31. https://doi.org/10.3390/pollutants2010004
Clergeaud F, Fagervold SK, Rodrigues AMS, Thorel E, Stien D, Lebaron P. Transfer of 7 Organic UV Filters from Sediment to the Ragworm Hediste diversicolor: Bioaccumulation of Benzophenone-3 and Further Proof of Octocrylene Metabolism. Pollutants. 2022; 2(1):23-31. https://doi.org/10.3390/pollutants2010004
Chicago/Turabian StyleClergeaud, Fanny, Sonja K. Fagervold, Alice M. S. Rodrigues, Evane Thorel, Didier Stien, and Philippe Lebaron. 2022. "Transfer of 7 Organic UV Filters from Sediment to the Ragworm Hediste diversicolor: Bioaccumulation of Benzophenone-3 and Further Proof of Octocrylene Metabolism" Pollutants 2, no. 1: 23-31. https://doi.org/10.3390/pollutants2010004