Evaluation of Solvent Compatibilities for Headspace-SIFT-MS Analysis of Pharmaceutical Products
Abstract
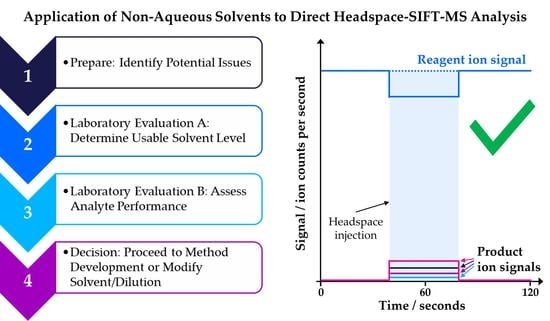
:1. Introduction
2. Materials and Methods
2.1. Automated SIFT-MS Analysis
2.2. SIFT-MS Analytical Method
2.3. Sample Preparation and Analysis
3. Results
3.1. Analyte Sensitivity as a Function of Diluent Solvent Proportion in Water
- Acetonitrile (and methanol, nitromethane): minimal change in sensitivity up to 10%.
- Tetrahydrofuran (THF) (and acetone, methyl ethyl ketone, propanal, isopropyl alcohol (IPA), 1-butanol): a small loss of sensitivity for each analyte compared to (1).
- Chloroform (and toluene, TCE): generally, further loss compared to (2), and significant drop in sensitivity when triacetin is no longer miscible in water (>6.1%).
3.2. Analytical Performance I: Linearity and Repeatability for Six Diluent Solvents
3.3. Analytical Performance II: LOQ and Recovery from DMI and DMI-Aqueous Systems
4. Discussion
4.1. Expanded Applicability of Headspace-SIFT-MS
4.2. Impact of Diluent on Specificity
- The SIFT-MS library records for diluent solvents describe their reaction chemistry for trace analysis and not that for solvent used as a bulk diluent. Hence, solvent product ions that are ordinarily inconsequential (e.g., product ion branching ratios of very low abundance—a few percent), can become significant.
- Isotopologue peaks, such as those arising from 13C and 18O, can be significant from bulk solvent and these are not usually recorded in the library.
- High-purity solvents can contain volatile impurities that may interfere with analytes.
- Benzene analysis with DMSO as diluent (Figure 5a): the solvent and analyte have identical product ions at unit mass resolution [16]. The two NO+ product ions for these compounds (Table 2 and Table S1) have different relative abundances and hence illustrate a general principle: interference will usually affect an analyte’s product ions differently. The ability to reliably quantify benzene in DMSO will depend on the benzene concentration and the proportion of DMSO diluent used;
- 1-Butanol analysis with diluent DMF (Table S5a and Figure S3): the 13C isotopologue of DMF interferes with the 1-butanol product ion. This isotopologue was previously observed to interfere with N-nitrosodimethylamine (NDMA) and the effect was mitigated through simple subtraction [43];
- Isooctane analysis is challenging in DMSO, triacetin, and DMI (Table 4), due to unidentified volatile impurities in these solvents. It is beyond the scope of this study to identify and mitigate these interferences (although for DMI, this might be a case of very low branching ratio product ion at MW–1). However, this step should be conducted as part of the evaluation workflow (Section 4.4), typically using full scan analyses of the solvent diluted in water.
4.3. Impact of Diluent on Sensitivity
4.4. A Workflow for Identifying and Evaluating Alternative Diluent Solvents
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| DIMS | direct-injection mass spectrometry |
| DMAC | N,N-dimethylacetamide |
| DMF | N,N-dimethylformamide |
| DMI | 1,3-dimethyl-2-imidazolidinone |
| DMSO | dimethyl sulfoxide |
| GC | gas chromatography |
| GC-FID | gas chromatography-flame ionization detection |
| HS-GC | headspace-gas chromatography |
| IPA | isopropyl alcohol |
| LC | liquid chromatography |
| LOQ | limit of quantitation |
| MEK | methyl ethyl ketone |
| PTR-MS | proton transfer reaction mass spectrometry |
| RSD | relative standard deviation |
| SIFT-MS | selected ion flow tube mass spectrometry |
| TCE | trichloroethylene |
| THF | tetrahydrofuran |
| USP | United States Pharmacopeia |
| VOC | volatile organic compound |
Appendix A. Additional Figures



References
- International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH). Impurities: Guideline for Residual Solvents Q3C(R8). 2021. Available online: https://database.ich.org/sites/default/files/ICH_Q3C-R8_Guideline_Step4_2021_0422_1.pdf (accessed on 23 March 2023).
- United States Pharmacopeia. Residual Solvents 〈467〉; United States Pharmacopeia: Rockville, MD, USA, 2007. [Google Scholar]
- United States Pharmacopeia. Residual Solvents—Verification of Compendial Procedures and Validation of Alternative Procedures 〈1467〉; United States Pharmacopeia: Rockville, MD, USA, 2019. [Google Scholar]
- International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH). ICH Guideline M7(R1) on Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk. 2017. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-m7r1-assessment-control-dna-reactive-mutagenic-impurities-pharmaceuticals-limit_en.pdf (accessed on 28 September 2022).
- European Medicines Agency. Questions and Answers for Marketing Authorisation Holders/Applicants on the CHMP Opinion for the Article 5(3) of Regulation (EC) No 726/2004 Referral on Nitrosamine Impurities in Human Medicinal Products, EMA/409815/2020. Available online: https://www.ema.europa.eu/en/documents/referral/nitrosamines-emea-h-a53-1490-questions-answers-marketing-authorisation-holders/applicants-chmp-opinion-article-53-regulation-ec-no-726/2004-referral-nitrosamine-impurities-human-medicinal-products_en.pdf (accessed on 15 September 2022).
- United States Food and Drug Administration. Guidance for Industry. Control of Nitrosamine Impurities in Human Drugs. 2021. Available online: https://www.fda.gov/media/141720/download (accessed on 15 September 2022).
- McEwan, M.J. Direct analysis mass spectrometry. In Ion Molecule Attachment Reactions: Mass Spectrometry; Fujii, T., Ed.; Springer: New York, NY, USA, 2015; pp. 263–317. [Google Scholar]
- Taylor, A.J.; Beauchamp, J.D.; Langford, V.S. Non-destructive and high-throughput—APCI-MS, PTR-MS and SIFT-MS as methods of choice for exploring flavor release. In Dynamic Flavor: Capturing Aroma Release Using Real-Time Mass Spectrometry; Beauchamp, J.D., Ed.; American Chemical Society: Washington DC, USA, 2021; pp. 1–16. [Google Scholar] [CrossRef]
- Smith, D.; McEwan, M.J.; Španěl, P. Understanding gas phase ion chemistry is the key to reliable selected ion flow tube-mass spectrometry analyses. Anal. Chem. 2020, 92, 12750–12762. [Google Scholar] [CrossRef] [PubMed]
- Biba, E.; Perkins, M.J.; Langford, V.S. Stimuli to the Revision Process: High-Throughput Residual Solvent Analysis Using Selected Ion Flow Tube Mass Spectrometry (SIFT-MS). U. S. Pharmacopeia. Pharm. Forum. 2021, 47, 1. Available online: https://online.usppf.com/usppf/document/GUID-2EE1BF6B-C82B-4F11-8E0B-C5520A4E8C3D_10101_en-US (accessed on 15 January 2023).
- Perkins, M.J.; Hastie, C.; Whitlock, S.E.; Langford, V.S. Pharmaceutical residual solvent analysis: A comparison of GC-FID and SIFT-MS performance. AppliedChem 2023, 3, 18. [Google Scholar] [CrossRef]
- Smith, D.; Španěl, P.; Demarais, N.; Langford, V.S.; McEwan, M.J. Recent developments and applications of selected ion flow tube mass spectrometry (SIFT-MS). Mass Spec. Rev. 2023, e21835. [Google Scholar] [CrossRef]
- Perkins, M.J.; Langford, V.S. Standard validation protocol for selected ion flow tube mass spectrometry methods applied to direct headspace analysis of aqueous volatile organic compounds. Anal Chem. 2021, 93, 8386–8392. [Google Scholar] [CrossRef] [PubMed]
- Perkins, M.J.; Langford, V.S. Application of headspace-SIFT-MS to direct analysis of hazardous volatiles in drinking water. Environments 2022, 9, 124. [Google Scholar] [CrossRef]
- Hastie, C.; Thompson, A.; Perkins, M.J.; Langford, V.S.; Eddleston, M.; Homer, N. Selected ion flow tube-mass spectrometry (SIFT-MS) as an alternative to gas chromatography/mass spectrometry (GC/MS) for the analysis of cyclohexanone and cyclohexanol in plasma. ACS Omega 2021, 6, 32818–32822. [Google Scholar] [CrossRef]
- Perkins, M.J.; Langford, V.S. Application of routine analysis procedures to a direct mass spectrometry technique: Selected ion flow tube mass spectrometry (SIFT-MS). Rev. Sep. Sci. 2021, 3, e21003. [Google Scholar] [CrossRef]
- Perkins, M.J. Methanolic Extraction of Soils by Automated Selected ion Flow Tube Mass Spectrometry (SIFT-MS). Anatune Application Note. Available online: http://bit.ly/3XeCaSI (accessed on 14 January 2023).
- Smith, D.; Španěl, P. Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis. Mass Spec. Rev. 2005, 24, 661–700. [Google Scholar] [CrossRef]
- Dai, L.; Quiroga, A.C.; Zhang, K.; Runes, H.B.; Yazzie, D.T.; Mistry, K.; Chetwyn, N.P.; Dong, M.W. A generic headspace GC method for residual solvents in pharmaceuticals: Benefits, rationale, and adaptations for new chemical entities. LCGC North Am. 2010, 28, 54–66. Available online: https://www.researchgate.net/publication/281259692 (accessed on 23 March 2023).
- Langford, V.S. SIFT-MS: Quantifying the volatiles you smell … and the toxics you don’t. Chemosensors 2023, 11, 111. [Google Scholar] [CrossRef]
- Hera, D.; Langford, V.S.; McEwan, M.J.; McKellar, T.I.; Milligan, D.B. Negative reagent ions for real time detection using SIFT-MS. Environments 2017, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Langford, V.S.; Billiau, C.; McEwan, M.J. Evaluation of the efficacy of SIFT-MS for speciation of wastewater treatment plant odors in parallel with human sensory analysis. Environments 2020, 7, 90. [Google Scholar] [CrossRef]
- Langford, V.S.; Du Bruyn, C.; Padayachee, D. An evaluation of selected ion flow tube mass spectrometry for rapid instrumental determination of paper type, origin and sensory attributes. Packag. Technol. Sci. 2021, 34, 245–260. [Google Scholar] [CrossRef]
- Prince, B.J.; Milligan, D.B.; McEwan, M.J. Application of selected ion flow tube mass spectrometry to real-time atmospheric monitoring. Rapid Commun. Mass Spectrom. 2010, 24, 1763–1769. [Google Scholar] [CrossRef]
- Wagner, R.L.; Farren, N.J.; Davison, J.; Young, S.; Hopkins, J.R.; Lewis, A.C.; Carslaw, D.C.; Shaw, M.D. Application of a mobile laboratory using a selected-ion flow-tube mass spectrometer (SIFT-MS) for characterisation of volatile organic compounds and atmospheric trace gases. Atmos. Meas. Tech. 2021, 14, 6083–6100. [Google Scholar] [CrossRef]
- Syft Technologies Limited. SIFT-MS Compound Library; Syft Technologies Limited: Christchurch, New Zealand, 2006. [Google Scholar]
- Syft Technologies Limited. SIFT-MS Compound Library; Syft Technologies Limited: Christchurch, New Zealand, 2014. [Google Scholar]
- Perkins, M.J. Unpublished Kinetic Data for 1,3-Dimethyl-2-imidazolidinone; Element Materials Technology: Cambridge, UK, 2023; Data available on request. [Google Scholar]
- Španěl, P.; Smith, D. SIFT studies of the reactions of H3O+, NO+, and O2+• with a series of alcohols. Int. J. Mass Spectrom. Ion Proc. 1997, 167/168, 375–388. [Google Scholar] [CrossRef]
- Perkins, M.J. Unpublished Kinetic Data for Triacetin; Element Materials Technology: Cambridge, UK, 2023; Data available on request. [Google Scholar]
- Španěl, P.; Ji, Y.; Smith, D. SIFT studies of the reactions of H3O+, NO+, and O2+• with a series of aldehydes and ketones. Int. J. Mass Spectrom. Ion Proc. 1997, 165/166, 25–37. [Google Scholar] [CrossRef]
- Španěl, P.; Smith, D. Selected ion flow tube studies of the reactions of H3O+, NO+, and O2+• with several amines and some other nitrogen-containing molecules. Int. J. Mass Spectrom. 1998, 176, 203–211. [Google Scholar] [CrossRef]
- Španěl, P.; Smith, D. SIFT studies of the reactions of H3O+, NO+ and O2+• with several aromatic and aliphatic hydrocarbons. Int. J. Mass Spectrom. 1998, 181, 1–10. [Google Scholar] [CrossRef]
- Španěl, P.; Smith, D. Selected ion flow tube studies of the reactions of H3O+, NO+, and O2+• with some chloroalkanes and chloroalkenes. Int. J. Mass Spectrom. 1999, 184, 175–181. [Google Scholar] [CrossRef]
- Arnold, S.T.; Viggiano, A.A.; Morris, R.A. Rate constants and product branching fractions for the reactions of H3O+ and NO+ with C2−C12 Alkanes. J. Phys. Chem. A 1998, 102, 8881–8887. [Google Scholar] [CrossRef]
- Dryahina, K.; Polasek, M.; Španěl, P. A selected ion flow tube, SIFT, study of the ion chemistry of H3O+, NO+, and O2+• ions with several nitroalkanes in the presence of water vapour. Int. J. Mass Spectrom. 2004, 239, 57–65. [Google Scholar] [CrossRef]
- Španěl, P.; Smith, D. SIFT studies of the reactions of H3O+, NO+, and O2+• with several ethers. Int. J. Mass Spectrom Ion Proc. 1998, 172, 239–247. [Google Scholar] [CrossRef]
- Kolb, B.; Ettre, L.S. Static Headspace-Gas Chromatography—Theory and Practice; Wiley-VCH: New York, NY, USA, 1997. [Google Scholar]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 90th ed.; CRC Press: Boca Raton, FL, USA, 2009; ISBN 978-1-4200-9084-0. [Google Scholar]
- International Union of Pure and Applied Chemistry (IUPAC). Compendium of Analytical Nomenclature: Definitive Rules, Section 18.4.3.7. 2002. Available online: http://publications.iupac.org/analytical_compendium/Cha18sec437.pdf (accessed on 13 March 2021).
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29, S49–S52. [Google Scholar]
- LabSyft Software: Compound Library, Syft Technologies Limited: Christchurch, New Zealand, 2019.
- Perkins, M.J.; Langford, V.S. Simple, Rapid Analysis of N-Nitrosodimethylamine (NDMA) Impurity in Ranitidine Products Using SIFT-MS. Syft Technologies Application Note. 2022. Available online: http://bit.ly/3GB8wzW (accessed on 12 January 2023).









| Solvent Name | Acronym | Molecular Formula | Molecular Weight (MR)/g mol−1 | CAS Number |
|---|---|---|---|---|
| N,N-Dimethylacetamide | DMAC | C3H7NO | 87.12 | 127-19-5 |
| N,N-Dimethylformamide | DMF | C4H9NO | 73.09 | 68-12-2 |
| Dimethyl sulfoxide | DMSO | C2H6OS | 78.13 | 67-68-5 |
| 1,3-Dimethyl-2-imidazolidinone | DMI | C5H10N2O | 114.15 | 80-73-9 |
| Methanol | – | CH4O | 32.04 | 67-56-1 |
| Triacetin | – | C9H14O6 | 218.20 | 102-76-1 |
| Solvent | Reagent Ions and Product Ion m/z (Ion Signal Ratio) | Reference | ||
|---|---|---|---|---|
| H3O+ | NO+ | O2+• | ||
| N,N-Dimethylacetamide | 88 (100%) | 87 (85%) 117 (15%) | 43 (30%) 87 (70%) | [26] |
| N,N-Dimethylformamide | 29 (5%) 74 (95%) | 72 (45%) 73 (45%) 103 (10%) | 43 (5%) 73 (95%) | [26] |
| Dimethyl sulfoxide | 79 (100%) | 78 (95%) 108 (5%) | 79 (100%) | [27] |
| 1,3-Dimethyl-2-imidazolidinone | 115 (100%) | 114 (100%) | 113 (8%) 114 (92%) | [28] |
| Methanol | 33 (100%) | 62 (100%) | 31 (50%) 32 (50%) | [29] |
| Triacetin | 45 (20%) 59 (20%) 159 (60%) | 159 (33%) 248 (67%) | 103 (17%) 115 (16%) 116 (17%) 145 (50%) | [30] |
| Level Used Here | Mix 1 Solution Concentration/ppm | Mix 2 Solution Concentration (All Analytes)/ppm | ||||
|---|---|---|---|---|---|---|
| Benzene, Toluene, TCE | Chloroform, Isooctane, Propanal | THF, MEK | Acetone, Acetonitrile, IPA, Nitromethane | 1-Butanol, Methanol | ||
| Low Range | ||||||
| 1 | 0.0005 | 0.002 | 0.02 | 0.05 | 0.1 | 0.05 |
| 2 | 0.001 | 0.004 | 0.04 | 0.1 | 0.2 | 0.1 |
| 3 | 0.0015 | 0.006 | 0.06 | 0.15 | 0.3 | 0.15 |
| 5 | 0.0025 | 0.01 | 0.1 | 0.25 | 0.5 | 0.25 |
| 10 | 0.005 | 0.02 | 0.2 | 0.5 | 1.0 | 0.5 |
| 15 | 0.0075 | 0.03 | 0.3 | 0.75 | 1.5 | 0.75 |
| 20 | 0.01 | 0.04 | 0.4 | 1 | 2 | 1 |
| High Range | ||||||
| 20 | 0.01 | 0.04 | 0.4 | 1 | 2 | 1 |
| 50 | 0.025 | 0.1 | 1 | 2.5 | 5 | 2.5 |
| 100 | 0.05 | 0.2 | 2 | 5 | 10 | 5 |
| 200 | 0.1 | 0.4 | 4 | 10 | 20 | 10 |
| 250 | 0.125 | 0.5 | 5 | 12.5 | 25 | 12.5 |
| 300 | 0.15 | 0.6 | 6 | 15 | 30 | 15 |
| 400 | 0.2 | 0.8 | 8 | 20 | 40 | 20 |
| 500 | 0.25 | 1 | 10 | 25 | 50 | 25 |
| Solvent | Dilution Level in Water | Range of R2 Observed for High Range (Low Range) | Exceptions |
|---|---|---|---|
| Water 1 | – | 0.9978–0.9999 (0.9928–0.9996) | (Isooctane (H113 ion): 0.8150) |
| DMAC | 5% | 0.9911–0.9981 | |
| 10% | 0.9921–0.9992 | ||
| 25% | 0.9950–0.9999 | Isooctane (H113 ion): 0.9861 | |
| 50% | Polar: 0.9982–0.9998 Non-polar: 0.9399–0.9725 | Propanal: 0.9679 Benzene: 0.3622–0.9222; Isooctane (H113 ion): 0.8288 | |
| DMF | 5% | 0.9970–0.9999 | 1-Butanol: 0.9899 |
| DMI 4 | 5% | 0.9955–0.9998 | |
| (0.9909–0.9996) | 2 | ||
| 10% | 0.9939–0.9998 | Isooctane (H113 ion): 0.9713 | |
| (0.9901–0.9996) | 3 | ||
| 25% | 0.9953–0.9998 | Isooctane (H113 ion): 0.9857 | |
| 50% | Polar: 0.9984–0.9999 | Propanal: 0.9791 | |
| Non-polar: 0.9807–0.9982 | Benzene: 0.9660–0.9870 | ||
| 75% | Polar: 0.9980–0.9998 | Propanal: 0.9298 | |
| Non-polar: 0.9021–0.9791 | Isooctane: 0.6488 and 0.8483 | ||
| 100% | Polar: 0.9947–0.9999 | Propanal: 0.8844 | |
| Non-polar: 0.8459–0.9948 | Isooctane: 0.7414 and 0.8715 | ||
| DMSO | 5% | 0.9937–0.9999 | Benzene: 0.9685–0.9917 |
| 10% | 0.9929–0.9998 | Benzene: 0.9593–0.9943 | |
| 25% | 0.9919–0.9993 | Benzene: 0.0024–0.2291; Isooctane (H113 ion): 0.9865 | |
| Methanol | 5% | 0.9987–0.9999 | |
| Triacetin | 5% | 0.9961–0.9997 |
| Solvent | Dilution Level in Water | Analyte Level | ||
|---|---|---|---|---|
| 50 | 250 | 500 | ||
| Water | – | 1.2—2.6% | 2.1–6.9% | 0.93–2.7% |
| DMAC 1 | 5% | 2.1–7.6% | 1.3–6.3% | 1.2–4.5% |
| 10% | 1.7–8.9% | 4.3–10.0% | 2.2–8.0% | |
| 25% | 1.1–9.2% | 1.5–6.6% | 0.74–3.9% | |
| 50% | 1.1–8.1% | 2.8–9.1% | 1.9–7.8% | |
| DMF 2 | 5% | 1.5–6.5% | 0.66–7.0% | 0.55–4.6% |
| DMI 3 | 5% | 1.3–8.0% | 0.83–4.8% | 1.4–4.8% |
| 10% | 1.0–9.8% | 1.2–3.5% | 3.5–7.6% | |
| 25% | 2.7–7.7% | 2.1–4.3% | 3.5–7.6% | |
| 50% | 0.93–9.7% | 1.2–9.9% | 1.3–4.4% | |
| 75% | 1.1–3.8% | 1.1–4.3% | 0.77–7.2% | |
| 100% | 0.76–5.3% | 0.50–3.3% | 0.70–3.0% | |
| DMSO 4 | 5% | 1.3–6.1% | 1.6–8.9% | 1.8–5.6% |
| 10% | 5.4–8.7% | 0.55–6.4% | 0.57–2.9% | |
| 25% | 1.0–6.9% | 1.0–6.2% | 1.9–7.7% | |
| Methanol 5 | 5% | 1.2–5.6% | 1.6–5.0% | 2.2–17.1% |
| Triacetin | 5% | 1.3–9.6% | 0.91–5.1% | 0.63–5.4% |
| Proportion of DMI in Water | Mix No. | Limit of Quantitation (Expressed as Levels; Table 3) | Exceptions |
|---|---|---|---|
| 0% (Water) | 1 | 2 | − |
| 5% | 1 | 5 | Isooctane (both ions) |
| 10% | 1 | 5 | Benzene (N78); isooctane (H113) |
| 10% | 2 | 2 | − |
| 100% | 1 | Polar: 5 | Isopropyl alcohol (N59) |
| Non-polar: >20 | Toluene (both ions): level 10 | ||
| 100% | 2 | 5 | Chloroform (O85); isooctane (both ions); isopropyl alcohol (N59) |
| Solvent | Practical Reagent Ions | Limit * | Comments |
|---|---|---|---|
| DMAC | H3O+, NO+, O2+• | ≤50% | Impacts analysis of acetone |
| DMF | H3O+, NO+, O2+• | ≤10% | Impacts analysis of 1-butanol, butylamines, etc. |
| DMSO | H3O+, NO+, O2+• | ≤25% | Impacts analysis of benzene and isooctane |
| DMI | H3O+, NO+, O2+• | ≤100% | Watch for impurities in solvent, including adsorption from air |
| Methanol | NO+ | ≤20% | Useful if analyte(s) selectively analyzed using NO+ |
| Triacetin | H3O+, NO+, O2+• | <6% | Limited by miscibility in water |
| Desirable Diluent Properties | Solvent Reagent Ions and Product Ion m/z (Ion Signal Ratio) | |||||
|---|---|---|---|---|---|---|
| DMAC | DMI | DMF | DMSO | Methanol | Triacetin | |
| Low volatility | ✓ | ✓ | ✕ | ✓ | ||
| Miscibility in water | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Poor headspace partitioning from water | ✓ | ✓ | ✓ | ✓ | ✕ | ✓ |
| Low sensitivity of SIFT-MS reagent ion(s) 1 | ✓ | |||||
| Solvent purity 2 | ✓ | ✓ | ✓ | ✓ | ✓ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perkins, M.J.; Silva, L.P.; Langford, V.S. Evaluation of Solvent Compatibilities for Headspace-SIFT-MS Analysis of Pharmaceutical Products. Analytica 2023, 4, 313-335. https://doi.org/10.3390/analytica4030024
Perkins MJ, Silva LP, Langford VS. Evaluation of Solvent Compatibilities for Headspace-SIFT-MS Analysis of Pharmaceutical Products. Analytica. 2023; 4(3):313-335. https://doi.org/10.3390/analytica4030024
Chicago/Turabian StylePerkins, Mark J., Leslie P. Silva, and Vaughan S. Langford. 2023. "Evaluation of Solvent Compatibilities for Headspace-SIFT-MS Analysis of Pharmaceutical Products" Analytica 4, no. 3: 313-335. https://doi.org/10.3390/analytica4030024