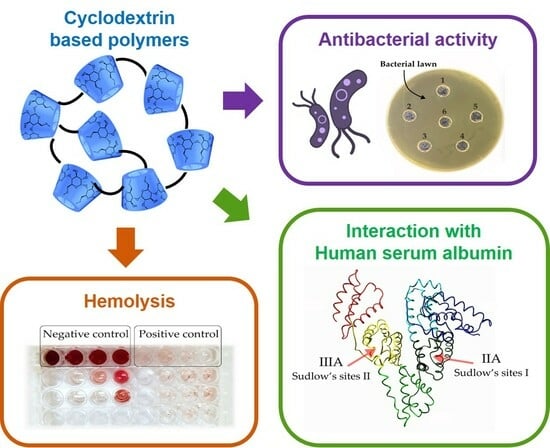
In Vitro Biological Properties of Cyclodextrin-Based Polymers: Interaction with Human Serum Albumin, Red Blood Cells and Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of HPCD and MCD Polymers Linked by Succinic Anhydride or 1,6-Hexamethylene Diisocyanate
2.2.2. FTIR-Spectroscopy
2.2.3. UV-Spectroscopy
2.2.4. Fluorescence Spectroscopy
2.2.5. NMR-Spectroscopy
2.2.6. Circular Dichroism Spectroscopy
2.2.7. Dynamic Light Scattering (DLS)
2.2.8. Nanoparticle Tracking Analysis (NTA)
2.2.9. Atomic Force Microscope
2.2.10. The CDpols Stability Studies
2.2.11. Hemolysis Assay
2.2.12. In Vitro Studies
3. Results and Discussion
3.1. Physico-Chemical Properties of CD-Based Polymers
3.2. The Structure of CDpols
3.3. Interaction of HSA with CD-Based Polymers
3.4. Influence of CD-Based Polymers on Binding HSA with Drugs
3.5. Blood Compatibility of CD-Polymers
3.6. Antibacterial Activity of CD-Polymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Ye, L.; Xi, J.; Wang, J.; Feng, Z. Cyclodextrin polymers: Structure, synthesis, and use as drug carriers. Prog. Polym. Sci. 2021, 118, 101408. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Bannerjee, S.; Bhati, L.; Pandey, S.; Pandey, P.; Sriwastawa, B. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef]
- Arslan, M.; Sanyal, R.; Sanyal, A. Cyclodextrin embedded covalently crosslinked networks: Synthesis and applications of hydrogels with nano-containers. Polym. Chem. 2020, 11, 615–629. [Google Scholar] [CrossRef]
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-based nanosponges: A critical review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef]
- Hashidzume, A.; Yamaguchi, H.; Harada, A. Cyclodextrin-Based Rotaxanes: From Rotaxanes to Polyrotaxanes and Further to Functional Materials. Eur. J. Org. Chem. 2019, 2019, 3344–3357. [Google Scholar] [CrossRef]
- Qie, S.; Hao, Y.; Liu, Z.; Wang, J.; Xi, J. Advances in Cyclodextrin Polymers and Their Applications in Biomedicine. Acta Chim. Sin. 2020, 78, 232. [Google Scholar] [CrossRef]
- Seidi, F.; Jin, Y.; Xiao, H. Polycyclodextrins: Synthesis, functionalization, and applications. Carbohydr. Polym. 2020, 242, 116277. [Google Scholar] [CrossRef]
- Yao, X.; Huang, P.; Nie, Z. Cyclodextrin-based polymer materials: From controlled synthesis to applications. Prog. Polym. Sci. 2019, 93, 1–35. [Google Scholar] [CrossRef]
- van de Manakker, F.; Vermonden, T.; van Nostrum, C.F.; Hennink, W.E. Cyclodextrin-Based Polymeric Materials: Synthesis, Properties, and Pharmaceutical/Biomedical Applications. Biomacromolecules 2009, 10, 3157–3175. [Google Scholar] [CrossRef] [PubMed]
- Simões, S.M.N.; Rey-Rico, A.; Concheiro, A.; Alvarez-Lorenzo, C. Supramolecular cyclodextrin-based drug nanocarriers. Chem. Commun. 2015, 51, 6275–6289. [Google Scholar] [CrossRef]
- Wankar, J.; Salzano, G.; Pancani, E.; Benkovics, G.; Malanga, M.; Manoli, F.; Gref, R.; Fenyvesi, E.; Manet, I. Efficient loading of ethionamide in cyclodextrin-based carriers offers enhanced solubility and inhibition of drug crystallization. Int. J. Pharm. 2017, 531, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Skuredina, A.A.; Le-Deygen, I.M.; Belogurova, N.G.; Kudryashova, E.V. Effect of cross-linking on the inclusion complex formation of derivatized β-cyclodextrins with small-molecule drug moxifloxacin. Carbohydr. Res. 2020, 498, 108183. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Paul, B.K.; Chattopadhyay, N. Interaction of cyclodextrins with human and bovine serum albumins: A combined spectroscopic and computational investigation. J. Chem. Sci. 2014, 126, 931–944. [Google Scholar] [CrossRef]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Aspects Med. 2012, 33, 209–290. [Google Scholar] [CrossRef]
- Skuredina, A.A.; Kopnova, T.Y.; Tychinina, A.S.; Golyshev, S.A.; Le-Deygen, I.M.; Belogurova, N.G.; Kudryashova, E.V. The New Strategy for Studying Drug-Delivery Systems with Prolonged Release: Seven-Day In Vitro Antibacterial Action. Molecules 2022, 27, 8026. [Google Scholar] [CrossRef]
- Yakupova, L.R.; Skuredina, A.A.; Markov, P.O.; Le-Deygen, I.M.; Kudryashova, E.V. Cyclodextrin Polymers as a Promising Drug Carriers for Stabilization of Meropenem Solutions. Appl. Sci. 2023, 13, 3608. [Google Scholar] [CrossRef]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lättekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowska, A.; Bhattacharjee, S.; et al. Zeta Potential of Extracellular Vesicles: Toward Understanding the Attributes that Determine Colloidal Stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef]
- Freichels, H.; Wagner, M.; Okwieka, P.; Meyer, R.G.; Mailänder, V.; Landfester, K.; Musyanovych, A. (Oligo)mannose functionalized hydroxyethyl starch nanocapsules: En route to drug delivery systems with targeting properties. J. Mater. Chem. B 2013, 1, 4338. [Google Scholar] [CrossRef] [PubMed]
- Paiphansiri, U.; Dausend, J.; Musyanovych, A.; Mailänder, V.; Landfester, K. Fluorescent Polyurethane Nanocapsules Prepared via Inverse Miniemulsion: Surface Functionalization for Use as Biocarriers. Macromol. Biosci. 2009, 9, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Aleem, O.; Kuchekar, B.; Pore, Y.; Late, S. Effect of β-cyclodextrin and hydroxypropyl β-cyclodextrin complexation on physicochemical properties and antimicrobial activity of cefdinir. J. Pharm. Biomed. Anal. 2008, 47, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhao, L.; Zhu, C.-S.; Huang, W.-Q.; Hu, J.-L. Water-insoluble β-cyclodextrin polymer crosslinked by citric acid: Synthesis and adsorption properties toward phenol and methylene blue. J. Incl. Phenom. Macrocycl. Chem. 2009, 63, 195–201. [Google Scholar] [CrossRef]
- Wolińska-Grabczyk, A.; Kaczmarczyk, B.; Jankowski, A. Investigations of hydrogen bonding in the poly(urethane-urea)-based membrane materials by using FTIR spectroscopy. PJCT 2008, 10, 53–56. [Google Scholar] [CrossRef]
- Stewart, J.E. Vibrational Spectra of Primary and Secondary Aliphatic Amines. J. Chem. Phys. 1959, 30, 1259–1265. [Google Scholar] [CrossRef]
- Baier, G.; Baumann, D.; Siebert, J.M.; Musyanovych, A.; Mailänder, V.; Landfester, K. Suppressing Unspecific Cell Uptake for Targeted Delivery Using Hydroxyethyl Starch Nanocapsules. Biomacromolecules 2012, 13, 2704–2715. [Google Scholar] [CrossRef]
- Kubczak, M.; Grodzicka, M.; Michlewska, S.; Karimov, M.; Ewe, A.; Aigner, A.; Bryszewska, M.; Ionov, M. The effect of novel tyrosine-modified polyethyleneimines on human albumin structure—Thermodynamic and spectroscopic study. Colloids Surf. B Biointerfaces 2023, 227, 113359. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Das, S.; Mukherjee, T.K. Insights into the Thermodynamics of Polymer Nanodot–Human Serum Albumin Association: A Spectroscopic and Calorimetric Approach. Langmuir 2016, 32, 12067–12077. [Google Scholar] [CrossRef]
- Suvarna, M.; Dyawanapelly, S.; Kansara, B.; Dandekar, P.; Jain, R. Understanding the Stability of Nanoparticle–Protein Interactions: Effect of Particle Size on Adsorption, Conformation and Thermodynamic Properties of Serum Albumin Proteins. ACS Appl. Nano Mater. 2018, 1, 5524–5535. [Google Scholar] [CrossRef]
- Treuel, L.; Malissek, M.; Gebauer, J.S.; Zellner, R. The Influence of Surface Composition of Nanoparticles on their Interactions with Serum Albumin. ChemPhysChem 2010, 11, 3093–3099. [Google Scholar] [CrossRef] [PubMed]
- Ragi, C.; Sedaghat-Herati, M.R.; Ouameur, A.A.; Tajmir-Riahi, H.A. The effects of poly(ethylene glycol) on the solution structure of human serum albumin. Biopolymers 2005, 78, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Tretiakova, D.; Le-Deigen, I.; Onishchenko, N.; Kuntsche, J.; Kudryashova, E.; Vodovozova, E. Phosphatidylinositol Stabilizes Fluid-Phase Liposomes Loaded with a Melphalan Lipophilic Prodrug. Pharmaceutics 2021, 13, 473. [Google Scholar] [CrossRef]
- Ghuman, J.; Zunszain, P.A.; Petitpas, I.; Bhattacharya, A.A.; Otagiri, M.; Curry, S. Structural Basis of the Drug-binding Specificity of Human Serum Albumin. J. Mol. Biol. 2005, 353, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, G.; Birkett, D.J.; Wade, D.N. The Characterization of Two Specific Drug Binding Sites on Human Serum Albumin. Mol. Pharmacol. 1975, 11, 824–832. [Google Scholar] [PubMed]
- Trynda-Lemiesz, L. Paclitaxel-HSA interaction. Binding sites on HSA molecule. Bioorganic Med. Chem. 2004, 12, 3269–3275. [Google Scholar] [CrossRef] [PubMed]
- Abu, T.M.M.; Ghithan, J.; Abu-Taha, M.I.; Darwish, S.M.; Abu-hadid, M.M. Spectroscopic approach of the interaction study of ceftriaxone and human serum albumin. J. Biophys. Struct. Biol. 2014, 6, 1–12. [Google Scholar] [CrossRef]
- Zhou, Z.; Hu, X.; Hong, X.; Zheng, J.; Liu, X.; Gong, D.; Zhang, G. Interaction characterization of 5−hydroxymethyl−2−furaldehyde with human serum albumin: Binding characteristics, conformational change and mechanism. J. Mol. Liq. 2020, 297, 111835. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, J.; Zhao, Y.; He, W.; Guo, Z. Noncovalent Interactions between a Trinuclear Monofunctional Platinum Complex and Human Serum Albumin. Inorg. Chem. 2011, 50, 12661–12668. [Google Scholar] [CrossRef]
- Marković, O.S.; Cvijetić, I.N.; Zlatović, M.V.; Opsenica, I.M.; Konstantinović, J.M.; Terzić Jovanović, N.V.; Šolaja, B.A.; Verbić, T.Ž. Human serum albumin binding of certain antimalarials. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 192, 128–139. [Google Scholar] [CrossRef]
- Hergert, L. Spectrofluorimetric study of the β-cyclodextrin–ibuprofen complex and determination of ibuprofen in pharmaceutical preparations and serum. Talanta 2003, 60, 235–246. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Blake, D.A.; Reed, W.F. Polydopamine particles as nontoxic, blood compatible, antioxidant and drug delivery materials. Colloids Surfaces B Biointerfaces 2018, 172, 618–626. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Aktas, N. Preparation of macro-, micro-, and nano-sized poly(Tannic acid) particles with controllable degradability and multiple biomedical uses. Polym. Degrad. Stab. 2016, 129, 96–105. [Google Scholar] [CrossRef]
- Szente, L.; Singhal, A.; Domokos, A.; Song, B. Cyclodextrins: Assessing the Impact of Cavity Size, Occupancy, and Substitutions on Cytotoxicity and Cholesterol Homeostasis. Molecules 2018, 23, 1228. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Skuredina, A.A.; Tychinina, A.S.; Le-Deygen, I.M.; Golyshev, S.A.; Kopnova, T.Y.; Le, N.T.; Belogurova, N.G.; Kudryashova, E.V. Cyclodextrins and Their Polymers Affect the Lipid Membrane Permeability and Increase Levofloxacin’s Antibacterial Activity In Vitro. Polymers 2022, 14, 4476. [Google Scholar] [CrossRef] [PubMed]







| CD Type | Linker | Abbreviation | ζ-Potential, mV |
|---|---|---|---|
| HPCD | succinic anhydride1, 6-hexamethylene diisocyanate | HPolS HPolH | –0.6 ± 0.1 13.8 ± 0.7 |
| MCD | succinic anhydride1, 6-hexamethylene diisocyanate | MPolS MPolH | 2.4 ± 1.2 31.6 ± 0.4 |
| α-Helix | β-Structures | Random Coil | |
|---|---|---|---|
| HSA | 62 ± 2 | 21 ± 1 | 17 ± 1 |
| HSA + HpolH | 60 ± 2 | 23 ± 1 | 17 ± 1 |
| HSA + HPolS | 59 ± 2 | 23± 1 | 18 ± 1 |
| HSA + MPolH | 59 ± 2 | 22 ± 1 | 19 ± 1 |
| HSA + MPolS | 59 ± 2 | 25 ± 1 | 16 ± 1 |
| Concentration, mg/mL | Type of Polymers | ||||
|---|---|---|---|---|---|
| HPolH | MPolH | HPolS | MPolS | HPolH–HPolS | |
| 3.30 | 1.9 ± 0.2 | 11.0 ± 0.5 | 2.4 ± 0.3 | 7.9 ± 0.4 | 0.6 ± 0.1 |
| 1.70 | 1.2 ± 0.1 | 4.2 ± 0.2 | 0 | 0 | 0.3 ± 0.1 |
| 0.17 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakupova, L.R.; Skuredina, A.A.; Kopnova, T.Y.; Kudryashova, E.V. In Vitro Biological Properties of Cyclodextrin-Based Polymers: Interaction with Human Serum Albumin, Red Blood Cells and Bacteria. Polysaccharides 2023, 4, 343-357. https://doi.org/10.3390/polysaccharides4040020
Yakupova LR, Skuredina AA, Kopnova TY, Kudryashova EV. In Vitro Biological Properties of Cyclodextrin-Based Polymers: Interaction with Human Serum Albumin, Red Blood Cells and Bacteria. Polysaccharides. 2023; 4(4):343-357. https://doi.org/10.3390/polysaccharides4040020
Chicago/Turabian StyleYakupova, Linara R., Anna A. Skuredina, Tatina Yu. Kopnova, and Elena V. Kudryashova. 2023. "In Vitro Biological Properties of Cyclodextrin-Based Polymers: Interaction with Human Serum Albumin, Red Blood Cells and Bacteria" Polysaccharides 4, no. 4: 343-357. https://doi.org/10.3390/polysaccharides4040020