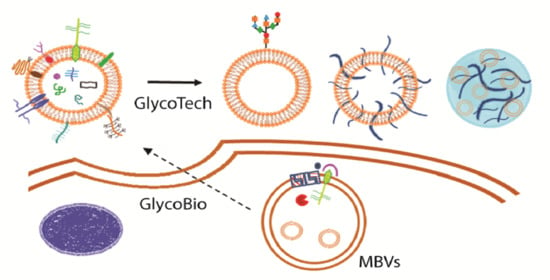
From Exosome Glycobiology to Exosome Glycotechnology, the Role of Natural Occurring Polysaccharides
Abstract
:1. Introduction
2. Glycobiology of Exosomes
2.1. Glycans
2.1.1. Glycan Role in Exosome Biology
2.1.2. Glycan Expression in Exosome Targeted Cells
3. Exosome Glycotechnology
3.1. Exosome: A Tunable Endogenous Nano-Delivery System
- (i)
- the endogenous origin results in a low immune response;
- (ii)
- the surface ligands and receptors expressed on the lipid membrane permit to easily pass through biofilm barrier and penetrate into target cells;
- (iii)
- the membrane bilayer structure effectively protects cargo from rapid degradation, increasing its delivery efficiency and enhancing the stability in plasma;
- (iv)
- the natural targeting ability enables the EXOs to migrate specifically in the target tissue.
3.2. Saccharides in Exosome-Based Delivery Systems
3.2.1. Exosome Glycocalyx for Targeted Delivery and Internalization
3.2.2. Polysaccharide Decoration for Controlled Biodistribution and Circulation Kinetics
4. Polysaccharide-Based Hydrogel for Sustained Exosome-Based Delivery System
- (i)
- in situ gelation: cargo is mixed into the polysaccharide viscous solution and, subsequently, a cross-linking agent is added to gel the system. Gelation can be achieved by ionic exchange, pH modification, temperature variation or UV irradiation.
- (ii)
- pre-formed gels: cargo is loaded directly in the polysaccharide-based hydrogel.
4.1. Polysaccharide-Based In Situ Gelling System, for a Sustained Delivery of Exosomes
4.2. Polysaccharide-Based Pre-Formed Hydrogels, for a Sustained Delivery of Exosomes
5. Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Buzas, E.I.; Toth, E.A.; Sodar, B.W.; Szabo-Taylor, K.E. Molecular interactions at the surface of extracellular vesicles. Semin. Immunopathol. 2018, 40, 453–464. [Google Scholar] [CrossRef] [Green Version]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Stahl, P.D.; Raposo, G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology 2019, 34, 169–177. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Schorey, J.S.; Harding, C.V. Extracellular vesicles and infectious diseases: New complexity to an old story. J. Clin. Investig. 2016, 126, 1181–1189. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yu, D. Exosomes in cancer development, metastasis, and immunity. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Rajakumar, G.; Venkidasamy, B.; Subramanian, U.; Thiruvengadam, M. Exosomes: Current use and future applications. Clin. Chim. Acta 2020, 500, 226–232. [Google Scholar] [CrossRef]
- Willms, E.; Cabanas, C.; Mager, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Ji, W.; Zhao, R.; Yang, J.; Lu, Z.; Li, Y.; Zhang, X. Exosome: A significant nano-scale drug delivery carrier. J. Mater. Chem. B 2020, 8, 7591–7608. [Google Scholar] [CrossRef]
- Jafari, D.; Shajari, S.; Jafari, R.; Mardi, N.; Gomari, H.; Ganji, F.; Forouzandeh Moghadam, M.; Samadikuchaksaraei, A. Designer Exosomes: A New Platform for Biotechnology Therapeutics. Bio Drugs 2020, 34, 567–586. [Google Scholar] [CrossRef]
- Cully, M. Exosome-based candidates move into the clinic. Nat. Rev. Drug Discov. 2021, 20, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Lin, Z.X.; Jiang, X.Y.; Yu, X.Y. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol. Sin. 2018, 39, 542–551. [Google Scholar] [CrossRef] [Green Version]
- Sallustio, F.; Curci, C.; Aloisi, A.; Toma, C.C.; Marulli, E.; Serino, G.; Cox, S.N.; De Palma, G.; Stasi, A.; Divella, C.; et al. Inhibin-A and Decorin Secreted by Human Adult Renal Stem/Progenitor Cells through the TLR2 Engagement Induce Renal Tubular Cell Regeneration. Sci. Rep. 2017, 7, 8225. [Google Scholar] [CrossRef] [Green Version]
- Toma, C.C.; Aloisi, A.; Bordoni, V.; Di Corato, R.; Rauner, M.; Cuniberti, G.; Delogu, L.G.; Rinaldi, R. Immune Profiling of Polysaccharide Submicron Vesicles. Biomacromolecules 2018, 19, 3560–3571. [Google Scholar] [CrossRef] [PubMed]
- García-Manrique, P.; Matos, M.; Gutiérrez, G.; Pazos, C.; Blanco-López, M.C. Therapeutic biomaterials based on extracellular vesicles: Classification of bio-engineering and mimetic preparation routes. J. Extracell. Vesicles 2018, 7, 1422676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of polysaccharide particle-based functional drug delivery. Carbohydr. Polym. 2019, 221, 94–112. [Google Scholar] [CrossRef]
- Lin, S.; Zhou, S.; Yuan, T. The “sugar-coated bullets” of cancer: Tumor-derived exosome surface glycosylation from basic knowledge to applications. Clin. Transl. Med. 2020, 10, e204. [Google Scholar] [CrossRef]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, C.; Royo, F.; Aizpurua-Olaizola, O.; Pazos, R.; Boons, G.J.; Reichardt, N.C.; Falcon-Perez, J.M. Glycosylation of extracellular vesicles: Current knowledge, tools and clinical perspectives. J. Extracell. Vesicles 2018, 7, 1442985. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Darvill, A.G.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H.; et al. Essentials of Glycobiology, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015–2017. [Google Scholar]
- McNaught, A.D.; Wilkinson, A. IUPAC. Compendium of Chemical Terminology, 2nd ed.; Blackwell Scientific: Oxford, UK, 1997. [Google Scholar]
- Schombs, M.; Gervay-Hague, J. Glycochemistry: Overview and Progress. In Glycochemical Synthesis; Shang-Cheng, H., Medel Manuel, L.Z., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2016; pp. 1–34. [Google Scholar]
- Davis, B.G. Sugars and proteins: New strategies in synthetic biology. Pure Appl. Chem. 2009, 81, 285–298. [Google Scholar] [CrossRef]
- Bertozzi, C.R.; Kiessling, L.L. Chemical glycobiology. Science 2001, 291, 2357–2364. [Google Scholar] [CrossRef] [Green Version]
- Lindahl, U.; Couchman, J.; Kimata, K.; Esko, J.D. Proteoglycans and Sulfated Glycosaminoglycans. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015; pp. 207–221. [Google Scholar]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Tsen, G.; Halfter, W.; Kroger, S.; Cole, G.J. Agrin is a heparan sulfate proteoglycan. J. Biol. Chem. 1995, 270, 3392–3399. [Google Scholar] [CrossRef] [Green Version]
- Bilandzic, M.; Stenvers, K.L. Betaglycan: A multifunctional accessory. Mol. Cell Endocrinol. 2011, 339, 180–189. [Google Scholar] [CrossRef]
- Seppinen, L.; Pihlajaniemi, T. The multiple functions of collagen XVIII in development and disease. Matrix Biol. 2011, 30, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Filmus, J.; Selleck, S.B. Glypicans: Proteoglycans with a surprise. J. Clin. Investig. 2001, 108, 497–501. [Google Scholar] [CrossRef]
- Kao, W.W.; Funderburgh, J.L.; Xia, Y.; Liu, C.Y.; Conrad, G.W. Focus on molecules: Lumican. Exp. Eye Res. 2006, 82, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Li, X.J.; Qian, C.N. Serglycin in human cancers. Chin. J. Cancer 2011, 30, 585–589. [Google Scholar] [CrossRef] [Green Version]
- Betriu, N.; Bertran-Mas, J.; Andreeva, A.; Semino, C.E. Syndecans and Pancreatic Ductal Adenocarcinoma. Biomolecules 2021, 11, 349. [Google Scholar] [CrossRef]
- Wight, T.N. Versican: A versatile extracellular matrix proteoglycan in cell biology. Curr. Opin. Cell Biol. 2002, 14, 617–623. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Kusuma, G.D.; Barabadi, M.; Tan, J.L.; Morton, D.A.V.; Frith, J.E.; Lim, R. To Protect and to Preserve: Novel Preservation Strategies for Extracellular Vesicles. Front. Pharmacol. 2018, 9, 1199. [Google Scholar] [CrossRef] [Green Version]
- Kalra, H.; Drummen, G.P.; Mathivanan, S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista, B.S.; Eng, W.S.; Pilobello, K.T.; Hendricks-Munoz, K.D.; Mahal, L.K. Identification of a conserved glycan signature for microvesicles. J. Proteome Res. 2011, 10, 4624–4633. [Google Scholar] [CrossRef] [Green Version]
- Escrevente, C.; Keller, S.; Altevogt, P.; Costa, J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer 2011, 11, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerezo-Magana, M.; Bang-Rudenstam, A.; Belting, M. The pleiotropic role of proteoglycans in extracellular vesicle mediated communication in the tumor microenvironment. Semin. Cancer Biol. 2020, 62, 99–107. [Google Scholar] [CrossRef]
- Martins, A.M.; Ramos, C.C.; Freitas, D.; Reis, C.A. Glycosylation of Cancer Extracellular Vesicles: Capture Strategies, Functional Roles and Potential Clinical Applications. Cells 2021, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Eng, W.S.; Colquhoun, D.R.; Dinglasan, R.R.; Graham, D.R.; Mahal, L.K. Complex N-linked glycans serve as a determinant for exosome/microvesicle cargo recruitment. J. Biol. Chem. 2014, 289, 32526–32537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, C.; Pazos, R.; Royo, F.; Gonzalez, E.; Roura-Ferrer, M.; Martinez, A.; Gamiz, J.; Reichardt, N.C.; Falcon-Perez, J.M. Assessing the role of surface glycans of extracellular vesicles on cellular uptake. Sci. Rep. 2019, 9, 11920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimoda, A.; Tahara, Y.; Sawada, S.I.; Sasaki, Y.; Akiyoshi, K. Glycan profiling analysis using evanescent-field fluorescence-assisted lectin array: Importance of sugar recognition for cellular uptake of exosomes from mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2017, 491, 701–707. [Google Scholar] [CrossRef]
- Klein-Scory, S.; Kubler, S.; Diehl, H.; Eilert-Micus, C.; Reinacher-Schick, A.; Stuhler, K.; Warscheid, B.; Meyer, H.E.; Schmiegel, W.; Schwarte-Waldhoff, I. Immunoscreening of the extracellular proteome of colorectal cancer cells. BMC Cancer 2010, 10, 70. [Google Scholar] [CrossRef] [Green Version]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.Y.; Dong, Y.P.; Sun, X.; Sui, X.; Zhu, H.; Zhao, Y.Q.; Zhang, Y.Y.; Mason, C.; Zhu, Q.; Han, S.X. High levels of serum glypican-1 indicate poor prognosis in pancreatic ductal adenocarcinoma. Cancer Med. 2018, 7, 5525–5533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frampton, A.E.; Prado, M.M.; Lopez-Jimenez, E.; Fajardo-Puerta, A.B.; Jawad, Z.A.R.; Lawton, P.; Giovannetti, E.; Habib, N.A.; Castellano, L.; Stebbing, J.; et al. Glypican-1 is enriched in circulating-exosomes in pancreatic cancer and correlates with tumor burden. Oncotarget 2018, 9, 19006–19013. [Google Scholar] [CrossRef] [Green Version]
- Hajrasouliha, A.R.; Jiang, G.; Lu, Q.; Lu, H.; Kaplan, H.J.; Zhang, H.G.; Shao, H. Exosomes from retinal astrocytes contain antiangiogenic components that inhibit laser-induced choroidal neovascularization. J. Biol. Chem. 2013, 288, 28058–28067. [Google Scholar] [CrossRef] [Green Version]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Roucourt, B.; Meeussen, S.; Bao, J.; Zimmermann, P.; David, G. Heparanase activates the syndecan-syntenin-ALIX exosome pathway. Cell Res. 2015, 25, 412–428. [Google Scholar] [CrossRef] [Green Version]
- Parimon, T.; Brauer, R.; Schlesinger, S.Y.; Xie, T.; Jiang, D.; Ge, L.; Huang, Y.; Birkland, T.P.; Parks, W.C.; Habiel, D.M.; et al. Syndecan-1 Controls Lung Tumorigenesis by Regulating miRNAs Packaged in Exosomes. Am. J. Pathol. 2018, 188, 1094–1103. [Google Scholar] [CrossRef] [Green Version]
- Ghossoub, R.; Chery, M.; Audebert, S.; Leblanc, R.; Egea-Jimenez, A.L.; Lembo, F.; Mammar, S.; Le Dez, F.; Camoin, L.; Borg, J.P.; et al. Tetraspanin-6 negatively regulates exosome production. Proc. Natl. Acad. Sci. USA 2020, 117, 5913–5922. [Google Scholar] [CrossRef] [PubMed]
- Christianson, H.C.; Svensson, K.J.; van Kuppevelt, T.H.; Li, J.P.; Belting, M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc. Natl. Acad. Sci. USA 2013, 110, 17380–17385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purushothaman, A.; Bandari, S.K.; Liu, J.; Mobley, J.A.; Brown, E.E.; Sanderson, R.D. Fibronectin on the Surface of Myeloma Cell-Derived Exosomes Mediates Exosome-Cell Interactions. J. Biol. Chem. 2016, 291, 1652–1663. [Google Scholar] [CrossRef] [Green Version]
- Uen, Y.; Wang, J.W.; Wang, C.; Jhang, Y.; Chung, J.Y.; Tseng, T.; Sheu, M.; Lee, S. Mining of potential microRNAs with clinical correlation-regulation of syndecan-1 expression by miR-122-5p altered mobility of breast cancer cells and possible correlation with liver injury. Oncotarget 2018, 9, 28165–28175. [Google Scholar] [CrossRef] [Green Version]
- Webber, J.; Steadman, R.; Mason, M.D.; Tabi, Z.; Clayton, A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010, 70, 9621–9630. [Google Scholar] [CrossRef] [Green Version]
- Purushothaman, A.; Bandari, S.K.; Chandrashekar, D.S.; Jones, R.J.; Lee, H.C.; Weber, D.M.; Orlowski, R.Z. Chondroitin sulfate proteoglycan serglycin influences protein cargo loading and functions of tumor-derived exosomes. Oncotarget 2017, 8, 73723–73732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Zhan, J.K.; Wang, Y.J.; Lin, X.; Zhong, J.Y.; Wang, Y.; Tan, P.; He, J.Y.; Cui, X.J.; Chen, Y.Y.; et al. Exosomes from hyperglycemia-stimulated vascular endothelial cells contain versican that regulate calcification/senescence in vascular smooth muscle cells. Cell Biosci. 2019, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.F.; Hua, K.; Woung, L.C.; Lin, C.H.; Chen, C.T.; Hsu, C.H.; Liou, S.W.; Tsai, C.Y. Expression Profiling of Exosomal miRNAs Derived from the Aqueous Humor of Myopia Patients. Tohoku J. Exp. Med. 2019, 249, 213–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT pathway. Dev. Cell 2011, 21, 77–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geminard, C.; De Gassart, A.; Blanc, L.; Vidal, M. Degradation of AP2 during reticulocyte maturation enhances binding of hsc70 and Alix to a common site on TFR for sorting into exosomes. Traffic 2004, 5, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Willms, E.; Johansson, H.J.; Mager, I.; Lee, Y.; Blomberg, K.E.; Sadik, M.; Alaarg, A.; Smith, C.I.; Lehtio, J.; El Andaloussi, S.; et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016, 6, 22519. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [Green Version]
- Thompson, C.A.; Purushothaman, A.; Ramani, V.C.; Vlodavsky, I.; Sanderson, R.D. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J. Biol. Chem. 2013, 288, 10093–10099. [Google Scholar] [CrossRef] [Green Version]
- Bang-Rudenstam, A.; Cerezo-Magana, M.; Belting, M. Pro-metastatic functions of lipoproteins and extracellular vesicles in the acidic tumor microenvironment. Cancer Metastasis Rev. 2019, 38, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Andreu, Z.; Yanez-Mo, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [Green Version]
- Guix, F.X.; Sannerud, R.; Berditchevski, F.; Arranz, A.M.; Horre, K.; Snellinx, A.; Thathiah, A.; Saido, T.; Saito, T.; Rajesh, S.; et al. Tetraspanin 6: A pivotal protein of the multiple vesicular body determining exosome release and lysosomal degradation of amyloid precursor protein fragments. Mol. Neurodegener. 2017, 12, 25. [Google Scholar] [CrossRef] [Green Version]
- Korpetinou, A.; Skandalis, S.S.; Labropoulou, V.T.; Smirlaki, G.; Noulas, A.; Karamanos, N.K.; Theocharis, A.D. Serglycin: At the crossroad of inflammation and malignancy. Front. Oncol. 2014, 3, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tao, J.; Li, Y.; Feng, Y.; Ju, H.; Wang, Z.; Ding, L. Quantitative Localized Analysis Reveals Distinct Exosomal Protein-Specific Glycosignatures: Implications in Cancer Cell Subtyping, Exosome Biogenesis, and Function. J. Am. Chem. Soc. 2020, 142, 7404–7412. [Google Scholar] [CrossRef]
- Chen, L.; Brigstock, D.R. Integrins and heparan sulfate proteoglycans on hepatic stellate cells (HSC) are novel receptors for HSC-derived exosomes. FEBS Lett. 2016, 590, 4263–4274. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.H.; Ketova, T.; Hoshino, D.; Zijlstra, A.; Weaver, A.M. Directional cell movement through tissues is controlled by exosome secretion. Nat. Commun. 2015, 6, 7164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alique, M.; Ruiz-Torres, M.P.; Bodega, G.; Noci, M.V.; Troyano, N.; Bohorquez, L.; Luna, C.; Luque, R.; Carmona, A.; Carracedo, J.; et al. Microvesicles from the plasma of elderly subjects and from senescent endothelial cells promote vascular calcification. Aging 2017, 9, 778–789. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Gao, W.; Zhang, Y.F.; Ho, M. Glypicans as Cancer Therapeutic Targets. Trends Cancer 2018, 4, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, C.G.; Diamandis, E.P. High throughput proteomic strategies for identifying tumour-associated antigens. Cancer Lett. 2007, 249, 110–119. [Google Scholar] [CrossRef]
- Sun, Z.; Shi, K.; Yang, S.; Liu, J.; Zhou, Q.; Wang, G.; Song, J.; Li, Z.; Zhang, Z.; Yuan, W. Effect of exosomal miRNA on cancer biology and clinical applications. Mol. Cancer 2018, 17, 147. [Google Scholar] [CrossRef]
- Lambaerts, K.; Wilcox-Adelman, S.A.; Zimmermann, P. The signaling mechanisms of syndecan heparan sulfate proteoglycans. Curr. Opin. Cell Biol. 2009, 21, 662–669. [Google Scholar] [CrossRef] [Green Version]
- Bunggulawa, E.J.; Wang, W.; Yin, T.; Wang, N.; Durkan, C.; Wang, Y.; Wang, G. Recent advancements in the use of exosomes as drug delivery systems. J. Nanobiotechnol. 2018, 16, 81. [Google Scholar] [CrossRef] [Green Version]
- Imai, T.; Takahashi, Y.; Nishikawa, M.; Kato, K.; Morishita, M.; Yamashita, T.; Matsumoto, A.; Charoenviriyakul, C.; Takakura, Y. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J. Extracell. Vesicles 2015, 4, 26238. [Google Scholar] [CrossRef]
- Wiklander, O.P.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charoenviriyakul, C.; Takahashi, Y.; Morishita, M.; Matsumoto, A.; Nishikawa, M.; Takakura, Y. Cell type-specific and common characteristics of exosomes derived from mouse cell lines: Yield, physicochemical properties, and pharmacokinetics. Eur. J. Pharm. Sci. 2017, 96, 316–322. [Google Scholar] [CrossRef]
- Saunderson, S.C.; Dunn, A.C.; Crocker, P.R.; McLellan, A.D. CD169 mediates the capture of exosomes in spleen and lymph node. Blood 2014, 123, 208–216. [Google Scholar] [CrossRef]
- Berenguer, J.; Lagerweij, T.; Zhao, X.W.; Dusoswa, S.; van der Stoop, P.; Westerman, B.; de Gooijer, M.C.; Zoetemelk, M.; Zomer, A.; Crommentuijn, M.H.W.; et al. Glycosylated extracellular vesicles released by glioblastoma cells are decorated by CCL18 allowing for cellular uptake via chemokine receptor CCR8. J. Extracell. Vesicles 2018, 7, 1446660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Royo, F.; Cossío, U.; Ruiz de Angulo, A.; Llop, J.; Falcon-Perez, J.M. Modification of the glycosylation of extracellular vesicles alters their biodistribution in mice. Nanoscale 2019, 11, 1531–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, J.P.K.; Stevens, M.M. Strategic design of extracellular vesicle drug delivery systems. Adv. Drug Deliv. Rev. 2018, 130, 12–16. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Zhao, M. Exosome-Based Cancer Therapy: Implication for Targeting Cancer Stem Cells. Front. Pharmacol. 2016, 7, 533. [Google Scholar] [CrossRef] [Green Version]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, H.X.; He, C.P.; Fan, S.; Zhu, Y.L.; Qi, C.; Huang, N.P.; Xiao, Z.D.; Lu, Z.H.; Tannous, B.A.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef]
- Smyth, T.; Petrova, K.; Payton, N.M.; Persaud, I.; Redzic, J.S.; Graner, M.W.; Smith-Jones, P.; Anchordoquy, T.J. Surface functionalization of exosomes using click chemistry. Bioconjug. Chem. 2014, 25, 1777–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pi, F.; Binzel, D.W.; Lee, T.J.; Li, Z.; Sun, M.; Rychahou, P.; Li, H.; Haque, F.; Wang, S.; Croce, C.M.; et al. Nanoparticle orientation to control RNA loading and ligand display on extracellular vesicles for cancer regression. Nat. Nanotechnol. 2018, 13, 82–89. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X.; Zhao, J.; Yang, Y.; Cai, X.; Xu, J.; Cao, P. Exosomes: A Novel Strategy for Treatment and Prevention of Diseases. Front. Pharmacol. 2017, 8, 300. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.T.; Umezaki, K.; Sawada, S.; Mukai, S.A.; Sasaki, Y.; Harada, N.; Shiku, H.; Akiyoshi, K. Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 2016, 6, 21933. [Google Scholar] [CrossRef] [Green Version]
- Kooijmans, S.A.A.; Schiffelers, R.M.; Zarovni, N.; Vago, R. Modulation of tissue tropism and biological activity of exosomes and other extracellular vesicles: New nanotools for cancer treatment. Pharmacol. Res. 2016, 111, 487–500. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, T.; Zhang, K.; Meng, X.; Dai, W.; Wang, D.; Dong, H.; Zhang, X. Engineered Exosome-Mediated Near-Infrared-II Region V(2)C Quantum Dot Delivery for Nucleus-Target Low-Temperature Photothermal Therapy. ACS Nano 2019, 13, 1499–1510. [Google Scholar] [CrossRef]
- Dusoswa, S.A.; Verhoeff, J.; Abels, E.; Méndez-Huergo, S.P.; Croci, D.O.; Kuijper, L.H.; de Miguel, E.; Wouters, V.; Best, M.G.; Rodriguez, E.; et al. Glioblastomas exploit truncated O-linked glycans for local and distant immune modulation via the macrophage galactose-type lectin. Proc. Natl. Acad. Sci. USA 2020, 117, 3693–3703. [Google Scholar] [CrossRef] [PubMed]
- Horrevorts, S.K.; Stolk, D.A.; van de Ven, R.; Hulst, M.; van Het Hof, B.; Duinkerken, S.; Heineke, M.H.; Ma, W.; Dusoswa, S.A.; Nieuwland, R.; et al. Glycan-Modified Apoptotic Melanoma-Derived Extracellular Vesicles as Antigen Source for Anti-Tumor Vaccination. Cancers 2019, 11, 1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.; Song, J.; Kang, Y.Y.; Mok, H. Mannose-Modified Serum Exosomes for the Elevated Uptake to Murine Dendritic Cells and Lymphatic Accumulation. Macromol. Biosci. 2019, 19, e1900042. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, H.; Noh, G.J.; Lee, E.S. pH-responsive hyaluronate-anchored extracellular vesicles to promote tumor-targeted drug delivery. Carbohydr. Polym. 2018, 202, 323–333. [Google Scholar] [CrossRef]
- Lee, H.; Park, H.; Yu, H.S.; Na, K.; Oh, K.T.; Lee, E.S. Dendritic Cell-Targeted pH-Responsive Extracellular Vesicles for Anticancer Vaccination. Pharmaceutics 2019, 11, 54. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Lee, H.; Youn, Y.S.; Oh, K.T.; Lee, E.S. Tumor-Homing pH-Sensitive Extracellular Vesicles for Targeting Heterogeneous Tumors. Pharmaceutics 2020, 12, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Ye, Z.; Xiang, M.; Chang, B.; Cui, J.; Ji, T.; Zhao, L.; Li, Q.; Deng, Y.; Xu, L.; et al. Functional extracellular vesicles engineered with lipid-grafted hyaluronic acid effectively reverse cancer drug resistance. Biomaterials 2019, 223, 119475. [Google Scholar] [CrossRef]
- Niu, W.; Xiao, Q.; Wang, X.; Zhu, J.; Li, J.; Liang, X.; Peng, Y.; Wu, C.; Lu, R.; Pan, Y.; et al. A Biomimetic Drug Delivery System by Integrating Grapefruit Extracellular Vesicles and Doxorubicin-Loaded Heparin-Based Nanoparticles for Glioma Therapy. Nano Lett. 2021, 21, 1484–1492. [Google Scholar] [CrossRef]
- Tamura, R.; Uemoto, S.; Tabata, Y. Augmented liver targeting of exosomes by surface modification with cationized pullulan. Acta Biomater. 2017, 57, 274–284. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.S.; Kim, Y.; Zhang, W.; Song, I.H.; Tung, C.H. Facile metabolic glycan labeling strategy for exosome tracking. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Altinoglu, S.; Takeda, Y.S.; Xu, Q. Integrating Protein Engineering and Bioorthogonal Click Conjugation for Extracellular Vesicle Modulation and Intracellular Delivery. PLoS ONE 2015, 10, e0141860. [Google Scholar] [CrossRef] [PubMed]
- Nishida-Aoki, N.; Tominaga, N.; Kosaka, N.; Ochiya, T. Altered biodistribution of deglycosylated extracellular vesicles through enhanced cellular uptake. J. Extracell. Vesicles 2020, 9, 1713527. [Google Scholar] [CrossRef]
- Nam, G.H.; Choi, Y.; Kim, G.B.; Kim, S.; Kim, S.A.; Kim, I.S. Emerging Prospects of Exosomes for Cancer Treatment: From Conventional Therapy to Immunotherapy. Adv. Mater. 2020, 32, e2002440. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Yuan, D.; Deygen, I.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: In vitro and in vivo evaluations. Nanomedicine 2018, 14, 195–204. [Google Scholar] [CrossRef]
- Antes, T.J.; Middleton, R.C.; Luther, K.M.; Ijichi, T.; Peck, K.A.; Liu, W.J.; Valle, J.; Echavez, A.K.; Marbán, E. Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. J. Nanobiotechnol. 2018, 16, 61. [Google Scholar] [CrossRef]
- Pezzana, C.; Agnely, F.; Bochot, A.; Siepmann, J.; Menasche, P. Extracellular Vesicles and Biomaterial Design: New Therapies for Cardiac Repair. Trends Mol. Med. 2021, 27, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yeo, Y. Controlled Drug Release from Pharmaceutical Nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Casu, B. Structure and biological activity of heparin. Adv. Carbohydr. Chem. Biochem. 1985, 43, 51–134. [Google Scholar] [PubMed]
- Oduah, E.I.; Linhardt, R.J.; Sharfstein, S.T. Heparin: Past, Present, and Future. Pharmaceuticals 2016, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Poletto, M.; Ornaghi, H.L.; Zattera, A.J. Native Cellulose: Structure, Characterization and Thermal Properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Farris, S.; Introzzi, L.; Fuentes-Alventosa, J.M.; Santo, N.; Rocca, R.; Piergiovanni, L. Self-assembled pullulan-silica oxygen barrier hybrid coatings for food packaging applications. J. Agric. Food Chem. 2012, 60, 782–790. [Google Scholar] [CrossRef] [Green Version]
- Prajapati, V.D.; Jani, G.K.; Khanda, S.M. Pullulan: An exopolysaccharide and its various applications. Carbohydr. Polym. 2013, 95, 540–549. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016, 6, 21961. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Wang, L.L.; Zaman, S.; Gordon, J.; Arisi, M.F.; Venkataraman, C.M.; Chung, J.J.; Hung, G.; Gaffey, A.C.; Spruce, L.A.; et al. Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction. Cardiovasc. Res. 2018, 114, 1029–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Yang, Y.; Li, Y.; Niu, X.; Zhao, B.; Wang, Y.; Bao, C.; Xie, Z.; Lin, Q.; Zhu, L. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 2017, 9, 4430–4438. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, X.; Chen, X.; Wei, Y.; Du, W.; Wang, Y.; Liu, L.; Zhao, W.; Han, Z.; Kong, D.; et al. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS Appl. Mater. Interfaces 2018, 10, 30081–30091. [Google Scholar] [CrossRef]
- Tao, S.C.; Guo, S.C.; Li, M.; Ke, Q.F.; Guo, Y.P.; Zhang, C.Q. Chitosan Wound Dressings Incorporating Exosomes Derived from MicroRNA-126-Overexpressing Synovium Mesenchymal Stem Cells Provide Sustained Release of Exosomes and Heal Full-Thickness Skin Defects in a Diabetic Rat Model. Stem. Cells Transl. Med. 2017, 6, 736–747. [Google Scholar] [CrossRef]
- Wang, C.; Liang, C.; Wang, R.; Yao, X.; Guo, P.; Yuan, W.; Liu, Y.; Song, Y.; Li, Z.; Xie, X. The fabrication of a highly efficient self-healing hydrogel from natural biopolymers loaded with exosomes for the synergistic promotion of severe wound healing. Biomater. Sci. 2019, 8, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ke, Q.F.; Tao, S.C.; Guo, S.C.; Rui, B.Y.; Guo, Y.P. Fabrication of hydroxyapatite/chitosan composite hydrogels loaded with exosomes derived from miR-126-3p overexpressed synovial mesenchymal stem cells for diabetic chronic wound healing. J. Mater. Chem. B 2016, 4, 6830–6841. [Google Scholar] [CrossRef]
- Guo, S.C.; Tao, S.C.; Yin, W.J.; Qi, X.; Yuan, T.; Zhang, C.Q. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics 2017, 7, 81–96. [Google Scholar] [CrossRef] [Green Version]
- Lv, K.; Li, Q.; Zhang, L.; Wang, Y.; Zhong, Z.; Zhao, J.; Lin, X.; Wang, J.; Zhu, K.; Xiao, C.; et al. Incorporation of small extracellular vesicles in sodium alginate hydrogel as a novel therapeutic strategy for myocardial infarction. Theranostics 2019, 9, 7403–7416. [Google Scholar] [CrossRef]
- Shafei, S.; Khanmohammadi, M.; Heidari, R.; Ghanbari, H.; Taghdiri Nooshabadi, V.; Farzamfar, S.; Akbariqomi, M.; Sanikhani, N.S.; Absalan, M.; Tavoosidana, G. Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: An in vivo study. J. Biomed. Mater. Res. A 2020, 108, 545–556. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, P.; Gao, X.; Chang, L.; Chen, Z.; Mei, X. Preparation of exosomes encapsulated nanohydrogel for accelerating wound healing of diabetic rats by promoting angiogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111671. [Google Scholar] [CrossRef]
- Xie, H.; Wang, Z.; Zhang, L.; Lei, Q.; Zhao, A.; Wang, H.; Li, Q.; Cao, Y.; Jie Zhang, W.; Chen, Z. Extracellular Vesicle-functionalized Decalcified Bone Matrix Scaffolds with Enhanced Pro-angiogenic and Pro-bone Regeneration Activities. Sci. Rep. 2017, 7, 45622. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, B.; Yin, P.; Zhao, L.; Wang, Y.; Fu, Z.; Dang, R.; Xu, J.; Zhang, J.; Wen, N. Integration of Human Umbilical Cord Mesenchymal Stem Cells-Derived Exosomes with Hydroxyapatite-Embedded Hyaluronic Acid-Alginate Hydrogel for Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 1590–1602. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.; Mu, J.; Chen, J.; Zhang, C.; Cao, H.; Gao, J. Transplantation of Human Mesenchymal Stem-Cell-Derived Exosomes Immobilized in an Adhesive Hydrogel for Effective Treatment of Spinal Cord Injury. Nano Lett. 2020, 20, 4298–4305. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Qian, Z.; Liu, D.; Sun, J.; Wang, X.; Liu, H.; Xu, J.; Guo, X. GMSC-Derived Exosomes Combined with a Chitosan/Silk Hydrogel Sponge Accelerates Wound Healing in a Diabetic Rat Skin Defect Model. Front. Physiol. 2017, 8, 904. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Wang, L.; Guan, J.; Tang, C.; He, N.; Zhang, W.; Fu, S. Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. Int. J. Biol. Macromol. 2018, 117, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, C.; Chen, M.; Xi, Y.; Cheng, W.; Mao, C.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; et al. Efficient Angiogenesis-Based Diabetic Wound Healing/Skin Reconstruction through Bioactive Antibacterial Adhesive Ultraviolet Shielding Nanodressing with Exosome Release. ACS Nano 2019, 13, 10279–10293. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, P.; Shangguan, L.; Ma, J.; Mao, K.; Zhang, Q.; Wang, Y.; Liu, Z.; Mao, K. A novel bacterial cellulose membrane immobilized with human umbilical cord mesenchymal stem cells-derived exosome prevents epidural fibrosis. Int. J. Nanomed. 2018, 13, 5257–5273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, F.; Zhang, D.; Fang, T.; Lu, C.; Wang, B.; Ding, X.; Wei, S.; Zhang, Y.; Pi, W.; Xu, H.; et al. Exosomes from Human Gingiva-Derived Mesenchymal Stem Cells Combined with Biodegradable Chitin Conduits Promote Rat Sciatic Nerve Regeneration. Stem Cells Int. 2019, 2019, 2546367. [Google Scholar] [CrossRef] [PubMed]





| Glycan | Molecular Type | Process | Glycan Function in Cell Pathway | Application | Ref. |
|---|---|---|---|---|---|
| High mannose, poly N-acetyllactosamine, α-2,6 sialic acid, complex N-linked glycan | Mannose repeated residues, repeated Galβ1-4GlcNAc disaccharides, alpha-keto acid sugar, oligosaccharide linked to nitrogen atom of protein | EXO molecular composition | Determining protein and glycosylated protein cargo in EXOs | Signaling for cell targeting | [42] |
| C-terminal of Agrin | Heparan-Sulfate PG | Basement membrane and EXO molecular composition | Tumor-associated antigen released in circulation | Autoantibodies diagnostic biomarker | [49] |
| Glypican | Heparan-Sulfate PG | EXO molecular composition | Membrane EXO composition | Diagnostic and prognostic biomarker | [50,51,52] |
| C-terminal Collagen XVIII (endostatin) | Heparan-Sulfate PG | ECM integrity | Blocking macrophage inflammation and vascular endothelial cell migration | Antiangiogenic therapy | [53] |
| Syndecan-1 | Heparan-Sulfate PG | EXO biogenesis | Molecular pathway activation in the complex syndecan-syntenin-ALIX-ESCRT | EXO release | [54,55] |
| Syndecan-1 | Heparan-Sulfate PG | EXO molecular composition and miRNA cargo modulation | Cell proliferation, ECM shaping | Signaling for cell targeting and diagnostic and prognostic marker for cancer | [56] |
| Syndecan-4 | Heparan-Sulfate PG | EXO biogenesis | Syndecan-4-tetraspanin-6 in regulation of EXO release ESCRT-independent | EXO release | [57] |
| Syndecan, Glypican | Heparan-Sulfate PG | EXO uptake | ERK1/2 cell signaling activation and cell migration | Drug delivery | [58] |
| Syndecan-1 | Heparan-Sulfate PG | EXO uptake | Bind of syndecan-1 with PG of target cell through ECM fibronectin (FN) | Cell-cell communication | [59] |
| Syndecan-1 | Heparan-Sulfate PG | EXO-hepatoma miRNA-122-5p regulates syndecan-1 expression in breast cancer cells | Activation of breast cancer cell motility and metastasis | Drug target for distant metastasis prevention | [60] |
| Betaglycan | Chondroitin-Heparan-Sulfate PG | TGF-β binding in EXO surface | Fibroblast to myofibroblast differentiation | Therapy strategy targeting betaglycan in cancer-altered stroma | [61] |
| Serglycin | Chondroitin-Sulfate PG | EXO protein cargo modulation | Myeloma cells proliferation and macrophages migration | Cancer treatment | [62] |
| Versican | Chondroitin-Sulfate PG | Senescent endothelial cells - EXO versican localizes to the mitochondria of VSMCs | Alteration of mitochondrial membrane potential and vascular smooth muscle cells senescence/calcification | Therapeutics target in diabetic vascular damage | [63] |
| Lumican | Leucine-rich keratan sulfate PG | Aqueous humor-EXO-has-miR405b-5p regulates lumican expression | Organization of collagen fibrils in sclera | Studies for diagnosis, treatment and prognosis of myopia | [64] |
| Ligands | Methods | TargetCells/Tissues | Ref. |
|---|---|---|---|
| Sialic acid residues | Surface deglycosylation of mouse liver-derived EXOs with neuraminidase | Intravenous injection in mice | [90] |
| α-2,3- and α-2,6 linked sialic acid-capped complex, N-glycans and bi-antennary N-glycans | Removal of sialic acids Incorporation of dendritic cell-specific intercellular adhesion molecules for receptor-mediated glycan-dependent targeting | In vitro study on human glioblastoma and monocyte-derived dendritic cells | [102] |
| High-mannose glycans | Overexpression of high-mannose glycans on melanoma cell surface and on EVs derived after the induction of cell apoptosis | In vitro study on dendritic cells | [103] |
| α-D-mannose PEG | Surface modification with α-D-mannose and PEG via the incorporation of 1,2-distearoyl-sn-glycero-3-phosphoethanolamine into the lipid layer of the EXOs | In vitro study on dendritic cells | [104] |
| HA3-(diethylamino)propylamine (HDEA) | Anchor of HA grafted with HDEA to EV membrane Doxorubicin loading | In vitro study on KB and HCT-116 tumor cells In vivo model of tumor-bearing mice | [105] |
| HA3-(diethylamino)propylamine (HDEA), monophosphoryl lipid A (MPLA), and mucin 1 peptide (MUC1) | Anchor of HA grafted with HDEA, MLA, MUC1 | In vitro study on dendritic cells and CD8+ T-cell | [106] |
| HA pH-responsive 3-(diethylamino)propylamine (HDEA) | Anchor of HA grafted with HDEA to EV membrane Doxorubicin loading | In vitro study on BT-474 and SK-N-MC cells. | [107] |
| Lipidomimetic chain conjugated HA | Synthesis of HA derivative with octadecyl tails (lipHA) and insertion into the EVs membrane to generate lipHA-engineered EVs (lipHA-hEVs) Doxorubicin loading | In vitro study on drug resistant MCF7/ADR cells Preclinical multidrug tumor models | [108] |
| Heparin | Patching of doxorubicin-loaded heparin-based nanoparticles onto the surface of natural grapefruit EVs | Glioma tissue | [109] |
| Pullulan, Spermine | Pullulan cationization with spermine by an N,N0-carbonyldiimidazole (CDI) activation method Mixing of MSC-derived EXOs with the cationized pullulan to incorporate the polysaccharide within the EXO membrane | In vitro study on HepG2 cells In vivo mouse model of liver injury | [110] |
| Azide containing sugars | Incorporation of tetra-acetylated N-azidoacetyl-D-mannosamine into glycans Bioorthogonal click reaction to label azido-containing EXOs with azadibenzylcyclooctyne-fluorescent dyes | In vitro tracking and in vivo biodistribution | [111] |
| Tetraacetylated n-azidoacetyl-d-mannosamine (ManNAz) azido sugar | Incorporation of ManNAz into EXOs Bioorthogonal click conjugation to modify and functionalize EXOs with a fluorescent dye Biotinylation | B16F10 cells | [112] |
| Material | Method | TargetCells/Tissues | Application | Reference |
|---|---|---|---|---|
| Thiol-Modified HA Gelatin Heparin | Bone marrow stem cell (BMSC)-derived EXOs entrapment in a matrix of thiolated HA, gelatin, and heparin Gel crosslinking with polyethylene glycol diacrylate | Human bone marrow stromal stem cell and osteoblast In vivo rat model of calvarial defects | Bone regeneration | [128] |
| Adamantane-modified HA β-cyclodextrin-modified HA | EXOs isolated from bone marrow-derived endothelial progenitor cells entrapment in an injectable HA-based hydrogel | In vivo rat model of myocardial infarction | Cardiac regeneration | [129] |
| O-nitrobenzyl alcohol modified HA Gelatin | HiPSC-MSCs-derived EXOs entrapment in a matrix of O-nitrobenzyl alcohol moieties modified HA and gelatin Gel crosslinking with light irradiation | Chondrocytes and human bone marrow stromal stem cell In vivo rabbit model of articular cartilage defect | Cartilage regeneration | [130] |
| CS | Human placenta-derived MSC EXOs incorporation in a CS solution, then gelled by adding β-glycero phosphate | In vivo murine model of hindlimb ischemia | Tissue regeneration Angiogenesis | [131] |
| CS | EXOs derived from microRNA-126-overexpressing synovium MSCs incorporation in a CS solution, then gelled | Human dermal fibroblasts and human dermal microvascular endothelial cells In vivo diabetic ratmodel | Wound healing | [132] |
| Aldehyde modified methylcellulose (MC-CHO) CS grafted poly(ethylene glycol) (CS-g-PEG) | Placental MSC-derived EXOs incorporation in a solution of MC-CHO Addition of CS-g-PEG and gelation | In vivo diabetic mouse model | Wound healing | [133] |
| CS Hydroxyapatite | EXOs derived from miR-126-3p overexpressed synovial MSC addition in a CS solution containing Ca(NO3)2*4H2O and Na2HPO4*2H2O | Human dermal fibroblasts and human dermal microvascular endothelial cells In vivo diabetic rat model | Wound healing | [134] |
| SA | Platelet-rich plasma-derived EXOs incorporation in a solution of SA Gelation with CaCl2 | Endothelial cells and fibroblasts In vivo diabetic rat model | Wound healing | [135] |
| SA | Bone marrow MSC-derived small EVs addition in a solution of SA Gelation with CaCl2 | In vivo rat model of myocardial infarction | Tissue regeneration Angiogenesis | [136] |
| SA | Adipose-derived stem cells EVs entrapment in a solution of SA Gelation with CaCl2 | HUVECs In vivo rat model of full-thickness woun | Wound healing | [137] |
| SA Polyvinyl alcohol (PVA) | Human umbilical cord MSC-derived EXOs encapsulation in a solution of SA and PVA Gelation with CaCl2 and ultrasonication | HUVECs In vivo diabetic rat model | Wound healing | [138] |
| SA | MSC-derived EVs alone or together with MSC entrapment in a solution of SA Gelation with CaCl2 | In vivo nude mouse model of subcutaneous bone formation | Bone regeneration Angiogenesis | [139] |
| Aldehyde-modified SA, HA-adipic dihydrazide Hydroxyapatite | Human umbilical cord MSC-derived EXOs integration in formulation containing aldehyde-modified SA, HA-adipic dihydrazide and hydroxyapatite before the gelation | Murine calvariae preosteoblast cell line In vivo rat model of calvarial bone defect | Bone regeneration | [140] |
| Material | Method | Target Cells/Tissues | Application | Reference |
|---|---|---|---|---|
| Oxidized HA Pluronic F127 Poly-ε-lysine | Adipose-derived MSC EXOs loading in a hydrogel composed of Pluronic F127, oxidized HA and poly-ε-lysine, obtained by the Schiff base reaction and thermal-responsive sol–gel process | In vivo diabetic mouse model | Wound healing | [141] |
| Aldehyde-modified hyaluronic acid (HA-CHO) Adipodihydrazide-modified hyaluronic acid (HA-ADH) | Human placenta amniotic membrane MSC-derived EXOs encapsulation within a hydrogel composed of HA–CHO and HA–ADH, modified with the laminin-derived adhesive peptide PPFLMLLKGSTR | In vivo spinal cord injury ratmodel | Spinal cord regeneration | [142] |
| CS Silk fibroin | EXOs derived from gingival MSCs loading in a hydrogel sponge composed of CS and silk fibroin prepared by freeze-drying method | In vivo diabetic mouse model | Wound healing | [143] |
| CS Silk fibroin | Platelet-rich plasma EXOs loading in a hydrogel sponge composed of CS and silk fibroin | In vivo diabetic rat model | Wound healing | [144] |
| Pullulan | EXOs derived from MSCs loading into a scaffold fabricated by the reversible Schiff base reaction between Pluronic F127 grafting polyethylenimine and aldehyde pullulan | In vivo diabetic mouse model | Wound healing | [145] |
| Bacterial cellulose | Human umbilical cord MSCs-derived EXOs loading into a bacterial cellulose membrane | In vivo laminectomy rabbit model | Epidural fibrosis prevention | [146] |
| Chitin | EXOs from gingival MSCs combination with biodegradable chitin conduits | In vivo sciatic nerve defect rat model | Peripheral nerve regeneration | [147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Rosa, G.; Ruggeri, C.; Aloisi, A. From Exosome Glycobiology to Exosome Glycotechnology, the Role of Natural Occurring Polysaccharides. Polysaccharides 2021, 2, 311-338. https://doi.org/10.3390/polysaccharides2020021
Della Rosa G, Ruggeri C, Aloisi A. From Exosome Glycobiology to Exosome Glycotechnology, the Role of Natural Occurring Polysaccharides. Polysaccharides. 2021; 2(2):311-338. https://doi.org/10.3390/polysaccharides2020021
Chicago/Turabian StyleDella Rosa, Giulia, Clarissa Ruggeri, and Alessandra Aloisi. 2021. "From Exosome Glycobiology to Exosome Glycotechnology, the Role of Natural Occurring Polysaccharides" Polysaccharides 2, no. 2: 311-338. https://doi.org/10.3390/polysaccharides2020021