Design of LPSO Phases in Mg-Y-Ni Alloys to Impact Hydrogenation Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Alloy and Samples Preparation
2.2. Characterization Methods
2.3. Hydrogenation Experiments
3. Results and Discussion
3.1. Tailoring LPSO Phase Volume Fraction via Composition and Processing in Mg-Y-Ni Aloys
3.1.1. LPSO Phase Composition
3.1.2. Microstructure
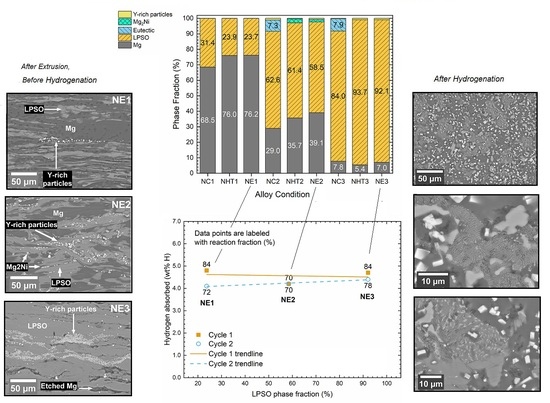
3.1.3. Phase Volume Fractions
3.1.4. X-ray Diffraction
3.2. The Effect of LPSO Phases on Hydrogenation Properties of Mg-Y-Ni Alloys
3.2.1. Absorption/Desorption Kinetics
3.2.2. Pressure–Composition Isotherms
3.2.3. XRD of Mg91Y5Ni4 before and after Hydrogen Experiments
3.2.4. Thin Foil Experiments
4. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yartys, V.; Lototskyy, M.; Akiba, E.; Albert, R.; Antonov, V.; Ares, J.; Baricco, M.; Bourgeois, N.; Buckley, C.; von Colbe, J.B.; et al. Magnesium based materials for hydrogen based energy storage: Past, present and future. Int. J. Hydrogen Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Rabkin, E.; Skripnyuk, V.; Estrin, Y. Ultrafine-Grained Magnesium Alloys for Hydrogen Storage Obtained by Severe Plastic Deformation. Front. Mater. 2019, 6, 240. [Google Scholar] [CrossRef]
- Wang, H.; Lin, H.; Cai, W.; Ouyang, L.; Zhu, M. Tuning kinetics and thermodynamics of hydrogen storage in light metal element based systems—A review of recent progress. J. Alloys Compd. 2016, 658, 280–300. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Huot, J.; Liang, G.; Boily, S.; Van Neste, A.; Schulz, R. Structural study and hydrogen sorption kinetics of ball-milled magnesium hydride. J. Alloys Compd. 1999, 293, 495–500. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Fujii, H. Catalytic effect of nanoparticle 3d-transition metals on hydrogen storage properties in magnesium hydride MgH2 prepared by mechanical milling. J. Phys. Chem. B 2005, 109, 7188–7194. [Google Scholar] [CrossRef]
- Yamasaki, N.; Miyazawa, H.; Ohyanagi, M.; Munir, Z.A. Accelerated hydrogen desorption from MgH2 by high-energy ball-milling with Al2O3. J. Mater. Sci. 2011, 47, 3577–3584. [Google Scholar] [CrossRef]
- Popilevsky, L.; Skripnyuk, V.; Beregovsky, M.; Sezen, M.; Amouyal, Y.; Rabkin, E. Hydrogen storage and thermal transport properties of pelletized porous Mg-2 wt.% multiwall carbon nanotubes and Mg-2 wt.% graphite composites. Int. J. Hydrogen Energy 2016, 41, 14461–14474. [Google Scholar] [CrossRef]
- Silva, R.; Neto, R.L.; Leiva, D.; Ishikawa, T.; Kiminami, C.; Jorge, A.; Botta, W. Room temperature hydrogen absorption by Mg and Mg TiFe nanocomposites processed by high-energy ball milling. Int. J. Hydrogen Energy 2018, 43, 12251–12259. [Google Scholar] [CrossRef]
- Alsabawi, K.; Gray, E.; Webb, C. The effect of ball-milling gas environment on the sorption kinetics of MgH2 with/without additives for hydrogen storage. Int. J. Hydrogen Energy 2019, 44, 2976–2980. [Google Scholar] [CrossRef]
- Kalinichenka, S.; Rontzsch, L.; Kieback, B. Structural and hydrogen storage properties of melt-spun Mg-Ni-Y alloys. Int. J. Hydrogen Energy 2009, 34, 7749–7755. [Google Scholar] [CrossRef]
- Bendersky, L.; Chiu, C.; Skripnyuk, V.; Rabkin, E. Effect of rapid solidification on hydrogen solubility in Mg-rich Mg–Ni alloys. Int. J. Hydrogen Energy 2011, 36, 5388–5399. [Google Scholar] [CrossRef]
- Leiva, D.R.; Costa, H.C.d.A.; Huot, J.; Pinheiro, T.S.; Junior, A.M.J.; Ishikawa, T.T.; Filho, W.J.B. Magnesium-Nickel alloy for hydrogen storage produced by melt spinning followed by cold rolling. Mater. Res. 2012, 15, 813–817. [Google Scholar] [CrossRef]
- Song, W.; Li, J.; Zhang, T.; Hou, X.; Kou, H. Dehydrogenation behavior and microstructure evolution of hydrogenated magnesium-nickel-yttrium melt-spun ribbons. RSC Adv. 2015, 5, 54258–54265. [Google Scholar] [CrossRef]
- Skripnyuk, V.M.; Rabkin, E.; Estrin, Y.; Lapovok, R. The effect of ball milling and equal channel angular pressing on the hydrogen absorption/desorption properties of Mg–4.95 wt% Zn–0.71 wt% Zr (ZK60) alloy. Acta Mater. 2004, 52, 405–414. [Google Scholar] [CrossRef]
- Leiva, D.R.; Huot, J.; Ishikawa, T.T.; Bolfarini, C.; Kiminami, C.S.; Jorge, A.M.; Filho, W.J.B. Hydrogen Activation Behavior of Commercial Magnesium Processed by Different Severe Plastic Deformation Routes. Mater. Sci. Forum 2010, 667–669, 1047–1051. [Google Scholar] [CrossRef]
- Jorge, A.M.; Prokofiev, E.; de Lima, G.F.; Rauch, E.; Veron, M.; Botta, W.J.; Kawasaki, M.; Langdon, T.G. An investigation of hydrogen storage in a magnesium-based alloy processed by equal-channel angular pressing. Int. J. Hydrogen Energy 2013, 38, 8306–8312. [Google Scholar] [CrossRef]
- Skryabina, N.; Aptukov, V.; Romanov, P.; Fruchart, D.; de Rango, P.; Girard, G.; Grandini, C.; Sandim, H.; Huot, J.; Lang, J.; et al. Microstructure Optimization of Mg-Alloys by the ECAP Process Including Numerical Simulation, SPD Treatments, Characterization, and Hydrogen Sorption Properties. Molecules 2018, 24, 89. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, J.; Ma, A.; Li, Y.; Song, D. A Critical Review of Mg-Based Hydrogen Storage Materials Processed by Equal Channel Angular Pressing. Metals 2017, 7, 324. [Google Scholar] [CrossRef]
- Liang, G.X.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. Catalytic effect of transition metals on hydrogen sorption in nanocrystalline ball milled MgH2-Tm (Tm = Ti, V, Mn, Fe and Ni) systems. J. Alloys Compd. 1999, 292, 247–252. [Google Scholar] [CrossRef]
- Cui, J.; Wang, H.; Liu, J.; Ouyang, L.; Zhang, Q.; Sun, D.; Yao, X.; Zhu, M. Remarkable enhancement in dehydrogenation of MgH2 by a nano-coating of multi-valence Ti-based catalysts. J. Mater. Chem. A 2013, 1, 5603–5611. [Google Scholar] [CrossRef]
- House, S.D.; Vajo, J.J.; Ren, C.; Rockett, A.A.; Robertson, I.M. Effect of ball-milling duration and dehydrogenation on the morphology, microstructure and catalyst dispersion in Ni-catalyzed MgH2 hydrogen storage materials. Acta Mater. 2015, 86, 55–68. [Google Scholar] [CrossRef]
- Tarasov, B.P.; Arbuzov, A.A.; Mozhzhuhin, S.A.; Volodin, A.A.; Fursikov, P.V.; Lototskyy, M.V.; Yartys, V.A. Hydrogen storage behavior of magnesium catalyzed by nickel-graphene nanocomposites. Int. J. Hydrogen Energy 2019, 44, 29212–29223. [Google Scholar] [CrossRef]
- Itoi, T.; Seimiya, T.; Kawamura, Y.; Hirohashi, M. Long period stacking structures observed in Mg97Zn1Y2 alloy. Scr. Mater. 2004, 51, 107–111. [Google Scholar] [CrossRef]
- Kawamura, Y.; Yamasaki, M. Formation and mechanical properties of Mg97Zn1RE2 alloys with long-period stacking ordered structure. Mater. Trans. 2007, 48, 2986–2992. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Morton, A.J.; Nie, J.F. The 18R and 14H long-period stacking ordered structures in Mg-Y-Zn alloys. Acta Mater. 2010, 58, 2936–2947. [Google Scholar] [CrossRef]
- Kishida, K.; Yokobayashi, H.; Inui, H.; Yamasaki, M.; Kawamura, Y. The crystal structure of the LPSO phase of the 14H-type in the Mg–Al–Gd alloy system. Intermetallics 2012, 31, 55–64. [Google Scholar] [CrossRef]
- Egusa, D.; Abe, E. The structure of long period stacking/order Mg–Zn–RE phases with extended non-stoichiometry ranges. Acta Mater. 2012, 60, 166–178. [Google Scholar] [CrossRef]
- Yamasaki, M.; Matsushita, M.; Hagihara, K.; Izuno, H.; Abe, E.; Kawamura, Y. Highly ordered 10H-type long-period stacking order phase in a Mg–Zn–Y ternary alloy. Scr. Mater. 2014, 78–79, 13–16. [Google Scholar] [CrossRef]
- Nie, J.F.; Zhu, Y.M.; Morton, A.J. On the Structure, Transformation and Deformation of Long-Period Stacking Ordered Phases in Mg-Y-Zn Alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2014, 45, 3338–3348. [Google Scholar] [CrossRef]
- Kishida, K.; Nagai, K.; Matsumoto, A.; Yasuhara, A.; Inui, H. Crystal structures of highly-ordered long-period stacking-ordered phases with 18R, 14H and 10H-type stacking sequences in the Mg–Zn–Y system. Acta Mater. 2015, 99, 228–239. [Google Scholar] [CrossRef]
- Oñorbe, E.; Garcés, G.; Pérez, P.; Adeva, P. Effect of the LPSO volume fraction on the microstructure and mechanical properties of Mg–Y2X–ZnX alloys. J. Mater. Sci. 2011, 47, 1085–1093. [Google Scholar] [CrossRef]
- Tong, L.; Li, X.; Zhang, D.; Cheng, L.; Meng, J.; Zhang, H. Dynamic recrystallization and texture evolution of Mg–Y–Zn alloy during hot extrusion process. Mater. Charact. 2014, 92, 77–83. [Google Scholar] [CrossRef]
- Drozdenko, D.; Farkas, G.; Šimko, P.; Fekete, K.; Čapek, J.; Garcés, G.; Ma, D.; An, K.; Máthis, K. Influence of Volume Fraction of Long-Period Stacking Ordered Structure Phase on the Deformation Processes during Cyclic Deformation of Mg-Y-Zn Alloys. Crystals 2020, 11, 11. [Google Scholar] [CrossRef]
- Hagihara, K.; Kinoshita, A.; Sugino, Y.; Yamasaki, M.; Kawamura, Y.; Yasuda, H.; Umakoshi, Y. Effect of long-period stacking ordered phase on mechanical properties of Mg97Zn1Y2 extruded alloy. Acta Mater. 2010, 58, 6282–6293. [Google Scholar] [CrossRef]
- Hagihara, K.; Li, Z.; Yamasaki, M.; Kawamura, Y.; Nakano, T. Strengthening mechanisms acting in extruded Mg-based long-period stacking ordered (LPSO)-phase alloys. Acta Mater. 2019, 163, 226–239. [Google Scholar] [CrossRef]
- Liu, J.; Zou, C.; Wang, H.; Ouyang, L.; Zhu, M. Facilitating de/hydrogenation by long-period stacking ordered structure in Mg based alloys. Int. J. Hydrogen Energy 2013, 38, 10438–10445. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Liu, B.; Lu, X.; Zhang, T.; Gu, Q. The cycling stability of the in situ formed Mg-based nanocomposite catalyzed by YH2. J. Mater. Chem. A 2017, 5, 17532–17543. [Google Scholar] [CrossRef]
- Lapovok, R.; Zolotoyabko, E.; Berner, A.; Skripnyuk, V.; Lakin, E.; Larianovsky, N.; Xu, C.; Rabkin, E. Hydrogenation effect on microstructure and mechanical properties of Mg-Gd-Y-Zn-Zr alloys. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2018, 719, 171–177. [Google Scholar] [CrossRef]
- Yang, T.; Li, Q.; Liang, C.; Wang, X.; Xia, C.; Wang, H.; Yin, F.; Zhang, Y. Microstructure and hydrogen absorption/desorption properties of Mg24Y3M (M = Ni, Co, Cu, Al) alloys. Int. J. Hydrogen Energy 2018, 43, 8877–8887. [Google Scholar] [CrossRef]
- Hagihara, K.; Kinoshita, A.; Sugino, Y.; Yamasaki, M.; Kawamura, Y.; Yasuda, H.; Umakoshi, Y. Plastic deformation behavior of Mg89Zn4Y7 extruded alloy composed of long-period stacking ordered phase. Intermetallics 2010, 18, 1079–1085. [Google Scholar] [CrossRef]
- Itoi, T.; Takahashi, K.; Moriyama, H.; Hirohashi, M. A high-strength Mg–Ni–Y alloy sheet with a long-period ordered phase prepared by hot-rolling. Scr. Mater. 2008, 59, 1155–1158. [Google Scholar] [CrossRef]
- Yang, X.; Wu, S.; Lü, S.; Hao, L.; Fang, X. Effects of Ni levels on microstructure and mechanical properties of Mg-Ni-Y alloy reinforced with LPSO structure. J. Alloys Compd. 2017, 726, 276–283. [Google Scholar] [CrossRef]
- Klebanoff, L.E.; Ott, K.C.; Simpson, L.J.; O’malley, K.; Stetson, N.T. Accelerating the Understanding and Development of Hydrogen Storage Materials: A Review of the Five-Year Efforts of the Three DOE Hydrogen Storage Materials Centers of Excellence. Met. Mater. Trans. E 2014, 1, 81–117. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, D.; Wang, Q.; Fang, F.; Sun, D.; Ouyang, L.; Zhu, M. Superior hydrogen storage kinetics of Mg12YNi alloy with a long-period stacking ordered phase. Scr. Mater. 2011, 65, 233–236. [Google Scholar] [CrossRef]
- Li, Y.; Gu, Q.; Li, Q.; Zhang, T. In-situ synchrotron X-ray diffraction investigation on hydrogen-induced decomposition of long period stacking ordered structure in Mg–Ni–Y system. Scr. Mater. 2017, 127, 102–107. [Google Scholar] [CrossRef]
- Song, W.; Dong, H.; Zhang, G.; Liu, J.; Yang, G.; Liu, Y.; Li, Y.; Li, J.; Shen, J.; Chen, Y.; et al. Enhanced hydrogen absorption kinetics by introducing fine eutectic and long-period stacking ordered structure in ternary eutectic Mg–Ni–Y alloy. J. Alloys Compd. 2019, 820, 153187. [Google Scholar] [CrossRef]
- Xie, L.; Xu, M. Improved Absorption and Desorption Kinetics of Mg–Ni–Ce Alloy Activated under Elevated Hydrogen Pressure. Mater. Trans. 2020, 61, 534–539. [Google Scholar] [CrossRef]
- Si, T.; Liu, Y.; Zhang, Q. Hydrogen storage properties of the supersaturated Mg12YNi solid solution. J. Alloys Compd. 2010, 507, 489–493. [Google Scholar] [CrossRef]
- Nicholson, K.S. Controlling long period stacking ordered phases in magnesium rare-earth alloys to alter hydrogenation behavior. In Institute for Frontier Materials; Deakin University: Geelong, VIC, Australia, 2021; p. 261. Available online: https://hdl.handle.net/10779/DRO/DU:21366234.v1 (accessed on 6 September 2023).
- Nicholson, K.S.; Skripnyuk, V.; Xu, C.; Gao, X.; Rabkin, E.; Hodgson, P.D.; Lapovok, R. Tailoring LPSO phases in Mg–Y–Zn alloys to govern hydrogenation kinetics. J. Mater. Sci. 2023, 58, 8572–8596. [Google Scholar] [CrossRef]
- Liu, C.; Luo, Q.; Gu, Q.-F.; Li, Q.; Chou, K.-C. Thermodynamic assessment of Mg−Ni−Y system focusing on long-period stacking ordered phases in the Mg-rich corner. J. Magnes. Alloys 2021, 10, 3250–3266. [Google Scholar] [CrossRef]
- Yamashita, K.; Itoi, T.; Yamasaki, M.; Kawamura, Y.; Abe, E. A novel long-period stacking/order structure in Mg-Ni-Y alloys. J. Alloys Compd. 2019, 788, 277–282. [Google Scholar] [CrossRef]
- Stampfer, J.F., Jr.; Holley, C.E., Jr.; Stuttle, J.F. The Magnesium-Hydrogen System. J. Am. Chem. Soc. 1960, 82, 3504–3508. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, Q.; Chen, S.; Chou, K.C.; Li, Q. Experimental investigation and thermodynamic calculation of the Mg–Ni–Y system (Y<50 at.%) at 400 and 500 °C. J. Alloys Compd. 2015, 649, 1306–1314. [Google Scholar]
- Jin, Q.-Q.; Fang, C.-F.; Mi, S.-B. Formation of long-period stacking ordered structures in Mg88M5Y7 (M=Ti, Ni and Pb) casting alloys. J. Alloys Compd. 2013, 568, 21–25. [Google Scholar] [CrossRef]
- Kishida, K.; Nagai, K.; Matsumoto, A.; Inui, H. Data in support of crystal structures of highly-ordered long-period stacking-ordered phases with 18R, 14H and 10H-type stacking sequences in the Mg–Zn–Y system. Data Brief 2015, 5, 314–320. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, S.; Bi, Y.; Li, H.; Ren, Y.; Qin, G. Phase equilibria of the long-period stacking ordered phase in the Mg–Ni–Y system. Intermetallics 2015, 57, 127–132. [Google Scholar] [CrossRef]
- Rokhlin, L.L. Corrosion resistance of magnesium alloys with rare earth metals. In Magnesium Alloys Containing Rare Earth metals: Structure and Properties; Taylor & Francis: London, UK; New York, NY, USA, 2003; p. 205. [Google Scholar]
- Liu, H.; Ju, J.; Yang, X.; Yan, J.; Song, D.; Jiang, J.; Ma, A. A two-step dynamic recrystallization induced by LPSO phases and its impact on mechanical property of severe plastic deformation processed Mg97Y2Zn1 alloy. J. Alloys Compd. 2017, 704, 509–517. [Google Scholar] [CrossRef]
- Kalinichenka, S.; Röntzsch, L.; Riedl, T.; Weißgärber, T.; Kieback, B. Hydrogen storage properties and microstructure of melt-spun Mg90Ni8RE2 (RE = Y, Nd, Gd). Int. J. Hydrogen Energy 2011, 36, 10808–10815. [Google Scholar] [CrossRef]
- Xie, L.; Xu, M.; Zhang, C.; Wu, T. Composition dependent hydrogen storage performance and desorption factors of Mg–Ce based alloys. Int. J. Hydrogen Energy 2020, 45, 9865–9876. [Google Scholar] [CrossRef]
- Xie, L.; Li, J.; Zhang, T.; Song, L.; Kou, H. Microstructure and hydrogen storage properties of Mg-Ni-Ce alloys with a long-period stacking ordered phase. J. Power Sources 2017, 338, 91–102. [Google Scholar] [CrossRef]
- Xie, L.; Li, J.; Zhang, T.; Kou, H. De/hydrogenation kinetics against air exposure and microstructure evolution during hydrogen absorption/desorption of Mg-Ni-Ce alloys. Renew. Energy 2017, 113, 1399–1407. [Google Scholar] [CrossRef]
- Ouyang, L.Z.; Yang, X.S.; Zhu, M.; Liu, J.W.; Dong, H.W.; Sun, D.L.; Zou, J.; Yao, X.D. Enhanced Hydrogen Storage Kinetics and Stability by Synergistic Effects of in Situ Formed CeH2.73 and Ni in CeH2.73-MgH2-Ni Nanocomposites. J. Phys. Chem. C 2014, 118, 7808–7820. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, D.; Wang, J.; Liu, B.; Peng, Q. Hydrogen storage properties of ultrahigh pressure Mg12NiY alloys with a superfine LPSO structure. Int. J. Hydrogen Energy 2019, 44, 23179–23187. [Google Scholar] [CrossRef]
- Yang, T.; Wang, P.; Li, Q.; Xia, C.; Yin, F.; Liang, C.; Zhang, Y. Hydrogen absorption and desorption behavior of Ni catalyzed Mg-Y-C Ni nanocomposites. Energy 2018, 165, 709–719. [Google Scholar] [CrossRef]
- Chiu, C.; Su, C.J.; Yu, W.H.; Rabkin, E. Microstructure and mechanical properties of Mg–GdH2 composite prepared by internal hydrogenation. J. Mater. Sci. 2022, 57, 11649–11662. [Google Scholar] [CrossRef]













| Alloy | Condition | Mg ± Std Err. | Ni ± Std Err. | Y ± Std Err. | Mg:Y | Mg:Ni | Ni:Y | Polytype |
|---|---|---|---|---|---|---|---|---|
| Mg97Y2Ni1 | As-cast | 89.36 ± 0.20 | 5.04 ± 0.11 | 5.60 ± 0.10 | 16.00 | 17.82 | 0.90 | 24R |
| Heat-treated | 86.43 ± 0.27 | 6.61 ± 0.13 | 6.96 ± 0.14 | 12.46 | 13.14 | 0.95 | 18R | |
| Extruded | 86.50 ± 0.19 | 6.72 ± 0.11 | 6.78 ± 0.09 | 12.80 | 12.92 | 0.99 | 18R | |
| Mg93Y4Ni3 | As-cast | 87.63 ± 0.45 | 6.44 ± 0.24 | 5.93 ± 0.21 | 14.94 | 13.80 | 1.08 | 14H |
| Heat-treated | 87.17 ± 0.20 | 6.72 ± 0.11 | 6.11 ± 0.10 | 14.31 | 13.02 | 1.10 | 14H | |
| Extruded | 86.99 ± 0.19 | 6.81 ± 0.08 | 6.20 ± 0.11 | 14.08 | 12.80 | 1.10 | 14H | |
| Mg91Y5Ni4 | As-cast | 83.89 ± 0.34 | 8.25 ± 0.17 | 7.86 ± 0.16 | 10.71 | 10.21 | 1.05 | 10H |
| 87.65 ± 0.14 | 6.37 ± 0.06 | 5.99 ± 0.10 | 14.67 | 13.78 | 1.06 | 14H | ||
| Heat-treated | 87.35 ± 0.07 | 6.41 ± 0.04 | 6.23 ± 0.05 | 14.07 | 14.02 | 1.03 | 14H | |
| 87.51 ± 0.16 | 6.25 ± 0.07 | 6.23 ± 0.09 | 14.02 | 13.63 | 1.00 | 14H | ||
| Extruded | 86.98 ± 0.11 | 6.72 ± 0.05 | 6.30 ± 0.06 | 13.83 | 12.96 | 1.07 | 14H |
| Composition * (Nominal/EDS) (at.%) | Absorption Plateau Pressure (Atm) | Desorption Plateau Pressure (Atm) | Maximum Hydrogen Capacity (wt.% H) | Reversible Hydrogen Capacity (wt.% H) | Calculated Capacity (wt.% H) |
|---|---|---|---|---|---|
| Mg97Y2Ni1/ Mg96.2Y2.2Ni1.6 | 1.6 ± 0.3 | 1.6 ± 0.2 | 5.7 | 5.2 | 7.0 ± 0.2 |
| Mg93Y4Ni3/ Mg91.1Y3.7Ni4.8 | 1.5 ± 0.5 | 1.5 ± 0.3 | 6.0 | 5.3 | 6.1 ± 0.2 |
| Mg91Y5Ni4/ Mg87.1Y6.7Ni6.2 | 1.8 ± 0.4 | 1.8 ± 0.2 | 5.6 | 4.5 | 5.9 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicholson, K.S.; Skripnyuk, V.; Xu, C.; Gao, X.; Rabkin, E.; Hodgson, P.D.; Lapovok, R. Design of LPSO Phases in Mg-Y-Ni Alloys to Impact Hydrogenation Kinetics. Hydrogen 2023, 4, 658-678. https://doi.org/10.3390/hydrogen4030042
Nicholson KS, Skripnyuk V, Xu C, Gao X, Rabkin E, Hodgson PD, Lapovok R. Design of LPSO Phases in Mg-Y-Ni Alloys to Impact Hydrogenation Kinetics. Hydrogen. 2023; 4(3):658-678. https://doi.org/10.3390/hydrogen4030042
Chicago/Turabian StyleNicholson, Kyle S., Vladimir Skripnyuk, Chunjie Xu, Xiang Gao, Eugen Rabkin, Peter D. Hodgson, and Rimma Lapovok. 2023. "Design of LPSO Phases in Mg-Y-Ni Alloys to Impact Hydrogenation Kinetics" Hydrogen 4, no. 3: 658-678. https://doi.org/10.3390/hydrogen4030042