Journal Description
Hydrogen
Hydrogen
is an international, peer-reviewed, open access journal on all aspects of hydrogen published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within ESCI (Web of Science), Scopus, CAPlus / SciFinder, and other databases.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 14.4 days after submission; acceptance to publication is undertaken in 2.9 days (median values for papers published in this journal in the second half of 2023).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
Latest Articles
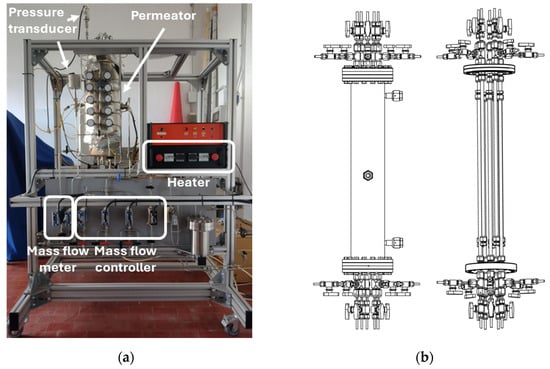
On the Scalability of a Membrane Unit for Ultrapure Hydrogen Separation
Hydrogen 2024, 5(2), 149-162; https://doi.org/10.3390/hydrogen5020010 - 17 Apr 2024
Abstract
►
Show Figures
Hydrogen permeation sparked a renewed interest in the second half of the 20th century due to the favorable features of this element as an energy factor. Furthermore, niche applications such as nuclear fusion gained attention for the highest selectivity ensured by self-supported dense
[...] Read more.
Hydrogen permeation sparked a renewed interest in the second half of the 20th century due to the favorable features of this element as an energy factor. Furthermore, niche applications such as nuclear fusion gained attention for the highest selectivity ensured by self-supported dense metallic membranes, especially those consisting of Pd-based alloys. In this framework, the ENEA Frascati laboratories have decades of experience in the manufacturing, integration, and operation of Pd-Ag permeators. Most of the experimental investigations were performed on single-tube membranes, proving their performance under relevant operational conditions. Nowadays, once the applicability of this technology has been demonstrated, the scalability of the single-tube experience over medium- and large-scale units must be verified. To do this, ENEA Frascati laboratories have designed and constructed a multi-tube permeator, namely the Medium-Scaled Membrane Reactor (MeSMeR), focused on scalability assessment. In this work, the results obtained with the MeSMeR facility have been compared with previous experimental campaigns conducted on single-tube units, and the scalability of the permeation results has been proven. Moreover, post-test simulations have been performed based on single-tube finite element modeling, proving the scalability of the numerical outcomes and the possibility of using this tool for scale-up design procedures.
Full article
Open AccessArticle
Effect of Metal Carbides on Hydrogen Embrittlement: A Density Functional Theory Study
by
Omar Faye and Jerzy A. Szpunar
Hydrogen 2024, 5(1), 137-148; https://doi.org/10.3390/hydrogen5010009 - 20 Mar 2024
Abstract
This study uses plane wave density functional theory (DFT) to investigate the effect of certain metal carbides (Niobium carbide, Vanadium carbide, Titanium carbide, and Manganese sulfide) on hydrogen embrittlement in pipeline steels. Our results predict that the interaction of hydrogen molecules with these
[...] Read more.
This study uses plane wave density functional theory (DFT) to investigate the effect of certain metal carbides (Niobium carbide, Vanadium carbide, Titanium carbide, and Manganese sulfide) on hydrogen embrittlement in pipeline steels. Our results predict that the interaction of hydrogen molecules with these metal carbides occurs in the long range with binding energy varying in the energy window [0.043 eV to 0.70 eV].In addition, our study shows the desorption of H2 molecules from these metal carbides in the chemisorptions. Since atomic state hydrogen interacts with NbC, VC, TiC, and MnS to cause embrittlement, we classified the strength of the hydrogen trapping as TiC + H > VC + H > NbC + H> MnS + H. In addition, our study reveals that the carbon site is a more favorable hydrogen-trapping site than the metal one.
Full article
(This article belongs to the Special Issue Feature Papers in Hydrogen (Volume 2))
►▼
Show Figures

Figure 1
Open AccessArticle
Effect of Cr/Mn Addition in TiVNb on Hydrogen Sorption Properties: Thermodynamics and Phase Transition Study
by
Anis Bouzidi, Erik Elkaim, Vivian Nassif and Claudia Zlotea
Hydrogen 2024, 5(1), 123-136; https://doi.org/10.3390/hydrogen5010008 - 18 Feb 2024
Abstract
►▼
Show Figures
High-entropy alloys (HEAs) are a promising class of materials that can grant remarkable functional performances for a large range of applications due to their highly tunable composition. Among these applications, recently, bcc HEAs capable of forming fcc hydrides have been proposed as high-capacity
[...] Read more.
High-entropy alloys (HEAs) are a promising class of materials that can grant remarkable functional performances for a large range of applications due to their highly tunable composition. Among these applications, recently, bcc HEAs capable of forming fcc hydrides have been proposed as high-capacity hydrogen storage materials with improved thermodynamics compared to classical metal hydrides. In this context, a single-phase bcc (TiVNb)0.90Cr0.05Mn0.05 HEA was prepared by arc melting to evaluate the effect of combined Cr/Mn addition in the ternary TiVNb. A thermodynamic destabilization of the fcc hydride phase was found in the HEA compared to the initial TiVNb. In situ neutron and synchrotron X-ray diffraction experiments put forward a fcc → bcc phase transition of the metallic subnetwork in the temperature range of 260–350 °C, whereas the H/D subnetwork underwent an order → disorder transition at 180 °C. The absorption/desorption cycling demonstrated very fast absorption kinetics at room temperature in less than 1 min with a remarkable total capacity (2.8 wt.%) without phase segregation. Therefore, the design strategy consisting of small additions of non-hydride-forming elements into refractory HEAs allows for materials with promising properties for solid-state hydrogen storage to be obtained.
Full article
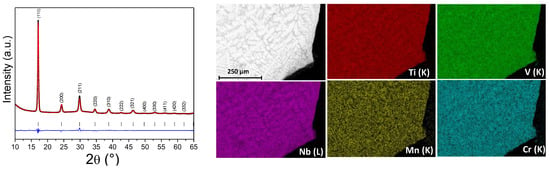
Figure 1
Open AccessArticle
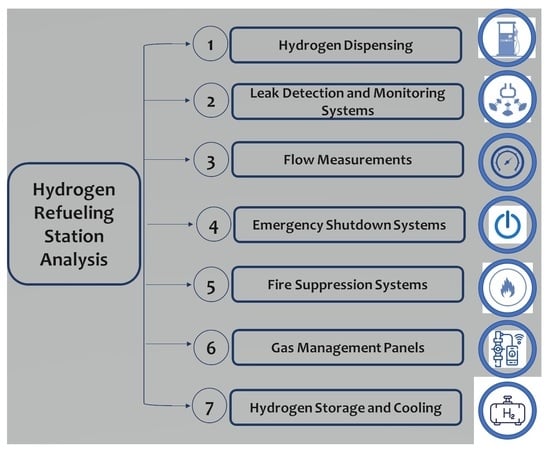
An Exploration of Safety Measures in Hydrogen Refueling Stations: Delving into Hydrogen Equipment and Technical Performance
by
Matteo Genovese, David Blekhman and Petronilla Fragiacomo
Hydrogen 2024, 5(1), 102-122; https://doi.org/10.3390/hydrogen5010007 - 17 Feb 2024
Cited by 1
Abstract
►▼
Show Figures
The present paper offers a thorough examination of the safety measures enforced at hydrogen filling stations, emphasizing their crucial significance in the wider endeavor to advocate for hydrogen as a sustainable and reliable substitute for conventional fuels. The analysis reveals a wide range
[...] Read more.
The present paper offers a thorough examination of the safety measures enforced at hydrogen filling stations, emphasizing their crucial significance in the wider endeavor to advocate for hydrogen as a sustainable and reliable substitute for conventional fuels. The analysis reveals a wide range of crucial safety aspects in hydrogen refueling stations, including regulated hydrogen dispensing, leak detection, accurate hydrogen flow measurement, emergency shutdown systems, fire-suppression mechanisms, hydrogen distribution and pressure management, and appropriate hydrogen storage and cooling for secure refueling operations. The paper therefore explores several aspects, including the sophisticated architecture of hydrogen dispensers, reliable leak-detection systems, emergency shut-off mechanisms, and the implementation of fire-suppression tactics. Furthermore, it emphasizes that the safety and effectiveness of hydrogen filling stations are closely connected to the accuracy in the creation and upkeep of hydrogen dispensers. It highlights the need for materials and systems that can endure severe circumstances of elevated pressure and temperature while maintaining safety. The use of sophisticated leak-detection technology is crucial for rapidly detecting and reducing possible threats, therefore improving the overall safety of these facilities. Moreover, the research elucidates the complexities of emergency shut-off systems and fire-suppression tactics. These components are crucial not just for promptly managing hazards, but also for maintaining the station’s structural soundness in unanticipated circumstances. In addition, the study provides observations about recent technical progress in the industry. These advances effectively tackle current safety obstacles and provide the foundation for future breakthroughs in hydrogen fueling infrastructure. The integration of cutting-edge technology and materials, together with the development of upgraded safety measures, suggests a positive trajectory towards improved efficiency, dependability, and safety in hydrogen refueling stations.
Full article
Graphical abstract
Open AccessReview
Hydrogen from Waste Gasification
by
Reinhard Rauch, Yohannes Kiros, Klas Engvall, Efthymios Kantarelis, Paulo Brito, Catarina Nobre, Santa Margarida Santos and Philipp A. Graefe
Hydrogen 2024, 5(1), 70-101; https://doi.org/10.3390/hydrogen5010006 - 10 Feb 2024
Cited by 1
Abstract
►▼
Show Figures
Hydrogen is a versatile energy vector for a plethora of applications; nevertheless, its production from waste/residues is often overlooked. Gasification and subsequent conversion of the raw synthesis gas to hydrogen are an attractive alternative to produce renewable hydrogen. In this paper, recent developments
[...] Read more.
Hydrogen is a versatile energy vector for a plethora of applications; nevertheless, its production from waste/residues is often overlooked. Gasification and subsequent conversion of the raw synthesis gas to hydrogen are an attractive alternative to produce renewable hydrogen. In this paper, recent developments in R&D on waste gasification (municipal solid waste, tires, plastic waste) are summarised, and an overview about suitable gasification processes is given. A literature survey indicated that a broad span of hydrogen relates to productivity depending on the feedstock, ranging from 15 to 300 g H2/kg of feedstock. Suitable gas treatment (upgrading and separation) is also covered, presenting both direct and indirect (chemical looping) concepts. Hydrogen production via gasification offers a high productivity potential. However, regulations, like frame conditions or subsidies, are necessary to bring the technology into the market.
Full article
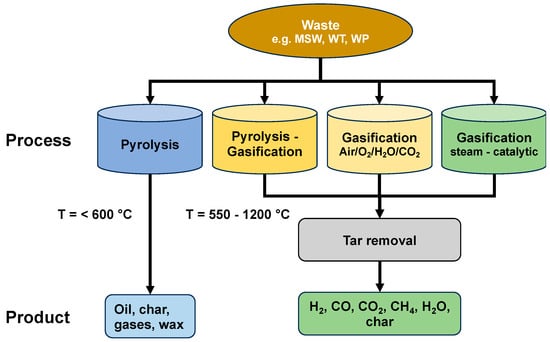
Figure 1
Open AccessCommunication
Techno-Economic Analysis of Cement Decarbonization Techniques: Oxygen Enrichment vs. Hydrogen Fuel
by
Bruno C. Domingues, Diogo M. F. Santos, Margarida Mateus and Duarte Cecílio
Hydrogen 2024, 5(1), 59-69; https://doi.org/10.3390/hydrogen5010005 - 10 Feb 2024
Abstract
►▼
Show Figures
The Paris Agreement aims to limit global warming, and one of the most polluting sectors is heavy industry, where cement production is a significant contributor. This work briefly explores some alternatives, recycling, reducing clinker content, waste heat recovery, and carbon capture, discussing their
[...] Read more.
The Paris Agreement aims to limit global warming, and one of the most polluting sectors is heavy industry, where cement production is a significant contributor. This work briefly explores some alternatives, recycling, reducing clinker content, waste heat recovery, and carbon capture, discussing their advantages and drawbacks. Then, it examines the economic viability and benefits of increasing oxygen concentration in the primary burning air from 21 to 27 vol.%, which could improve clinker production by 7%, and the production of hydrogen through PEM electrolysis to make up 5% of the fuel thermal fraction, considering both in a cement plant producing 3000 tons of clinker per day. This analysis used reference values from Secil, an international company for cement and building materials, to determine the required scale of the oxygen and hydrogen production, respectively, and calculate the CAPEX of each approach. It is concluded that oxygen enrichment can provide substantial fuel savings for a relatively low cost despite a possible significant increase in NOx emissions. However, hydrogen production at this scale is not currently economically viable.
Full article

Figure 1
Open AccessReview
The Use of Copper-Based Delafossite to Improve Hydrogen Production Performance: A Review
by
Hasnae Chfii, Amal Bouich and Bernabé Mari Soucase
Hydrogen 2024, 5(1), 39-58; https://doi.org/10.3390/hydrogen5010004 - 30 Jan 2024
Abstract
This review paper reports on the use of Delafossite as a layer between perovskite-based solar cells to improve hydrogen production efficiency and make the process easier. The investigation delves into the possible breakthroughs in sustainable energy generation by investigating the synergistic interplay between
[...] Read more.
This review paper reports on the use of Delafossite as a layer between perovskite-based solar cells to improve hydrogen production efficiency and make the process easier. The investigation delves into the possible breakthroughs in sustainable energy generation by investigating the synergistic interplay between Delafossite and solar technology. This investigation covers copper-based Delafossite material’s properties, influence on cell performance, and function in the electrolysis process for hydrogen production. Some reports investigate the synthesis and characterizations of delafossite materials and try to improve their performance using photo electrochemistry. This work sheds light on the exciting prospects of Delafossite integration using experimental and analytical methodologies.
Full article
(This article belongs to the Special Issue Feature Papers in Hydrogen (Volume 2))
►▼
Show Figures
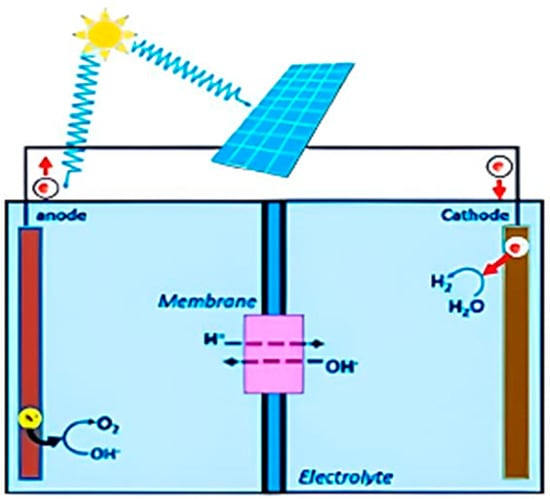
Figure 1
Open AccessArticle
Hydrogenation Thermodynamics of Ti16V60Cr24−xFex Alloys (x = 0, 4, 8, 12, 16, 20, 24)
by
Francia Ravalison and Jacques Huot
Hydrogen 2024, 5(1), 29-38; https://doi.org/10.3390/hydrogen5010003 - 26 Jan 2024
Abstract
The effect of the partial substitution of Cr with Fe on the thermodynamic parameters of vanadium-rich Ti16V60Cr24-xFex alloys (x = 0, 4, 8, 12, 16, 20, 24) was investigated. For each composition, a pressure–concentration isotherm (PCI)
[...] Read more.
The effect of the partial substitution of Cr with Fe on the thermodynamic parameters of vanadium-rich Ti16V60Cr24-xFex alloys (x = 0, 4, 8, 12, 16, 20, 24) was investigated. For each composition, a pressure–concentration isotherm (PCI) was registered at 298, 308, and 323 K. The PCI curves revealed a reduction in plateau pressure and a decrease in desorbed hydrogen capacity with an increasing amount of Fe. For all alloys, about 50% or less of the initial hydrogen capacity was desorbed for all chosen temperatures. Entropy (ΔS) and enthalpy (ΔH) values were deducted from corresponding Van’t Hoff plots of the PCI curves: the entropy values ranged from −150 to −57 J/K·mol H2, while the enthalpy values ranged from −44 to −21 kJ/mol H2. They both decreased with an increasing amount of Fe. Plotting ΔS as function of ΔH showed a linear variation that seems to indicate an enthalpy–entropy compensation. Moreover, a quality factor analysis demonstrated that the present relationship between entropy and enthalpy is not of a statistical origin at the 99% confidence level.
Full article
(This article belongs to the Topic Metal Hydrides: Fundamentals and Applications)
►▼
Show Figures
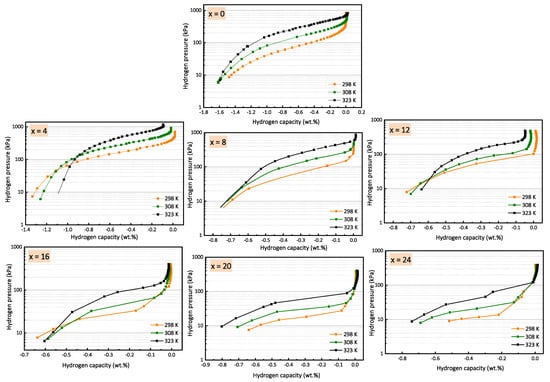
Figure 1
Open AccessArticle
Computational Modeling of High-Speed Flow of Two-Phase Hydrogen through a Tube with Abrupt Expansion
by
Konstantin I. Matveev
Hydrogen 2024, 5(1), 14-28; https://doi.org/10.3390/hydrogen5010002 - 18 Jan 2024
Abstract
►▼
Show Figures
Hydrogen can become a prevalent renewable fuel in the future green economy, but technical and economic hurdles associated with handling hydrogen must be overcome. To store and transport hydrogen in an energy-dense liquid form, very cold temperatures, around 20 K, are required. Evaporation
[...] Read more.
Hydrogen can become a prevalent renewable fuel in the future green economy, but technical and economic hurdles associated with handling hydrogen must be overcome. To store and transport hydrogen in an energy-dense liquid form, very cold temperatures, around 20 K, are required. Evaporation affects the achievable mass flow rate during the high-speed transfer of hydrogen at large pressure differentials, and accurate prediction of this process is important for the practical design of hydrogen transfer systems. Computational fluid dynamics modeling of two-phase hydrogen flow is carried out in the present study using the volume-of-fluid method and the Lee relaxation model for the phase change. Suitable values of the relaxation time parameter are determined by comparing numerical results with test data for high-speed two-phase hydrogen flows in a configuration involving a tube with sudden expansion, which is common in practical systems. Simulations using a variable outlet pressure are conducted to demonstrate the dependence of flow rates on the driving pressure differential, including the attainment of the critical flow regime. Also shown are computational results for flows with various inlet conditions and a fixed outlet state. Field distributions of the pressure, velocity, and vapor fractions are presented for several flow regimes.
Full article
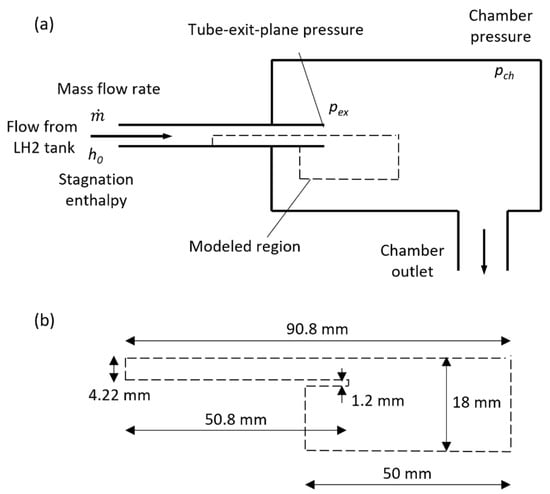
Figure 1
Open AccessArticle
Pt Effect on H2 Kinetics Sorption in Mn Oxide-Based Polymeric Material
by
Rolando Pedicini and Michalis Sigalas
Hydrogen 2024, 5(1), 1-13; https://doi.org/10.3390/hydrogen5010001 - 04 Jan 2024
Abstract
Recent studies have demonstrated how a material based on Mn oxide, supported by a polymeric matrix, shows an interesting H2 absorption capacity in non-drastic temperature and pressure conditions even if the reaction kinetics are particularly slow. In this study, therefore, two different
[...] Read more.
Recent studies have demonstrated how a material based on Mn oxide, supported by a polymeric matrix, shows an interesting H2 absorption capacity in non-drastic temperature and pressure conditions even if the reaction kinetics are particularly slow. In this study, therefore, two different percentages of Pt (5 and 10 wt%) were added to a composite sample, containing 50 wt% of Mn oxide, through a ball milling technique in order to verify the reduction in absorption kinetics of the quantity of added catalyst. The effect of the catalyst quantity on the composite matrix was investigated through morphological analyses of the SEM-EDX and TEM types, with which it was found that the distribution of Pt is more homogeneous compared to the sample containing 5%. XRD studies confirmed the simultaneous presence of the amorphous structure of the polymer and the crystalline structure of Pt, and absorption tests with the Sievert method verified a better kinetic reaction of the 10% Pt sample. In parallel, a modeling study, using the ab initio Density Functional Theory (DFT), was performed. The supercell for this study was Mn22Pt2O48. The number of H atoms gradually increased, starting from 2 (Mn22Pt2O48H2), where the initial desorption energy was 301 kJ/mol, to 211 kJ/mol for 12 H atoms (Mn22Pt2O48H12). From the experimental H2 absorption value (0.22 wt%), the number of respective H atoms was calculated (n = 5), and the corresponding desorption energy was equal to about 273 kJ/mol.
Full article
(This article belongs to the Special Issue Feature Papers in Hydrogen (Volume 2))
►▼
Show Figures
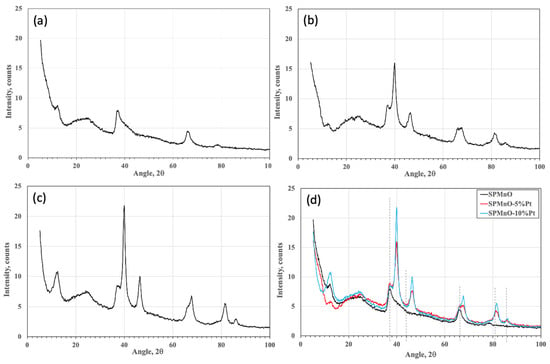
Figure 1
Open AccessArticle
In-Situ Hydrogen Charging Effect on the Fracture Behaviour of 42CrMo4 Steel Submitted to Various Quenched and Tempering Heat Treatments
by
Atif Imdad and Francisco Javier Belzunce Varela
Hydrogen 2023, 4(4), 1035-1050; https://doi.org/10.3390/hydrogen4040060 - 11 Dec 2023
Abstract
Research into safer, durable steels to be used in hydrogen-rich environments has been gaining importance in recent years. In this work, 42CrMo4 steel was subjected to quenched and tempered heat treatments using different temperature and time durations, in order to obtain different tempered
[...] Read more.
Research into safer, durable steels to be used in hydrogen-rich environments has been gaining importance in recent years. In this work, 42CrMo4 steel was subjected to quenched and tempered heat treatments using different temperature and time durations, in order to obtain different tempered martensite microstructures. Tensile tests on smooth and notched specimens were then performed in the air as well as with in situ electrochemical hydrogen charging using two different hydrogenated conditions. The harmful effects of hydrogen are more evident in tensile tests performed on notched specimens. The harder (stronger) the steel, the more hydrogen embrittlement occurs. As the steel’s internal local hydrogen concentration rises, its strength must be gradually reduced in order to choose the best steel. The observed embrittlement differences are explained by modifications in the operative failure micromechanisms. These change from ductile (microvoid coalescence) in the absence of hydrogen, or under low hydrogen levels in the case of the softest steels, to brittle (cleavage or even intergranular fracture) under the most severe conditions.
Full article
(This article belongs to the Topic Hydrogen—The New Energy Vector for the Transition of Industries "Hard to Abate")
►▼
Show Figures
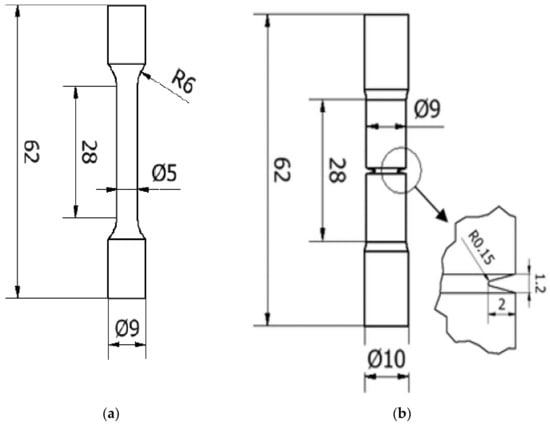
Figure 1
Open AccessArticle
Compression-Induced Dehydrogenation of Graphene: Insight from Simulations
by
Danil W. Boukhvalov and Vladimir Yu. Osipov
Hydrogen 2023, 4(4), 1022-1034; https://doi.org/10.3390/hydrogen4040059 - 09 Dec 2023
Abstract
►▼
Show Figures
In this work, we reported the results of systematic studies of various configurations of chemically adsorbed hydrogen atoms on the surface of corrugated graphene induced by in-plane uniaxial compression. Different magnitudes of the substrate corrugations have been considered. Results of the calculations demonstrate
[...] Read more.
In this work, we reported the results of systematic studies of various configurations of chemically adsorbed hydrogen atoms on the surface of corrugated graphene induced by in-plane uniaxial compression. Different magnitudes of the substrate corrugations have been considered. Results of the calculations demonstrate the visible difference in the electronic structure of corrugated non-hydrogenated graphene, contrary to the absence of a visible effect of corrugation of graphene. The reciprocal effect of corrugation and local hydrogenation on the permeation of protons (H+) throughout the graphene membrane is also discussed. Results of the periodic DFT calculations demonstrate that binding energy between graphene and large hydrogen clusters drastically decreases with increasing the magnitudes of the corrugation graphene substrate. A similar effect of decreasing hydrogen binding energies was also observed for corrugated graphane. The obtained results can be used to control the release of hydrogen from graphene by switching mechanical stress on and off without applying additional heat.
Full article
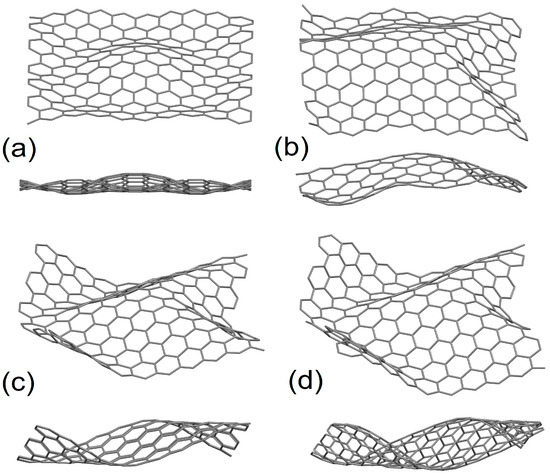
Figure 1
Open AccessArticle
CFD Simulations of Hydrogen Tank Fuelling: Sensitivity to Turbulence Model and Grid Resolution
by
Hanguang Xie, Dmitriy Makarov, Sergii Kashkarov and Vladimir Molkov
Hydrogen 2023, 4(4), 1001-1021; https://doi.org/10.3390/hydrogen4040058 - 06 Dec 2023
Cited by 1
Abstract
►▼
Show Figures
CFD modelling of compressed hydrogen fuelling provides information on the hydrogen and tank structure temperature dynamics required for onboard storage tank design and fuelling protocol development. This study compares five turbulence models to develop a strategy for cost-effective CFD simulations of hydrogen fuelling
[...] Read more.
CFD modelling of compressed hydrogen fuelling provides information on the hydrogen and tank structure temperature dynamics required for onboard storage tank design and fuelling protocol development. This study compares five turbulence models to develop a strategy for cost-effective CFD simulations of hydrogen fuelling while maintaining a simulation accuracy acceptable for engineering analysis: RANS models k-ε and RSM; hybrid models SAS and DES; and LES model. Simulations were validated against the fuelling experiment of a Type IV 29 L tank available in the literature. For RANS with wall functions and blended models with near-wall treatment, the simulated average hydrogen temperatures deviated from the experiment by 1–3% with CFL ≈ 1–3 and dimensionless wall distance y+ ≈ 50–500 in the tank. To provide a similar simulation accuracy, the LES modelling approach with near-wall treatment requires mesh with wall distance y+ ≈ 2–10 and demonstrates the best-resolved flow field with larger velocity and temperature gradients. LES simulation on this mesh, however, implies a ca. 60 times longer CPU time compared to the RANS modelling approach and 9 times longer compared to the hybrid models due to the time step limit enforced by the CFL ≈ 1.0 criteria. In all cases, the simulated pressure histories and inlet mass flow rates have a difference within 1% while the average heat fluxes and maximum hydrogen temperature show a difference within 10%. Compared to LES, the k-ε model tends to underestimate and DES tends to overestimate the temperature gradient inside the tank. The results of RSM and SAS are close to those of LES albeit of 8–9 times faster simulations.
Full article
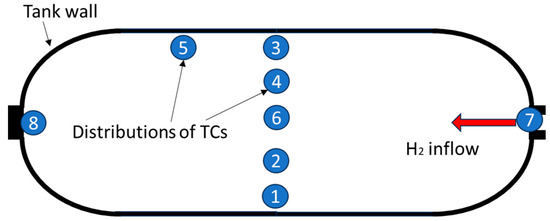
Figure 1
Open AccessReview
Technical and Economic Viability of Underground Hydrogen Storage
by
José Ernesto Quintos Fuentes and Diogo M. F. Santos
Hydrogen 2023, 4(4), 975-1000; https://doi.org/10.3390/hydrogen4040057 - 29 Nov 2023
Cited by 2
Abstract
►▼
Show Figures
Considering the mismatch between the renewable source availability and energy demand, energy storage is increasingly vital for achieving a net-zero future. The daily/seasonal disparities produce a surplus of energy at specific moments. The question is how can this “excess” energy be stored? One
[...] Read more.
Considering the mismatch between the renewable source availability and energy demand, energy storage is increasingly vital for achieving a net-zero future. The daily/seasonal disparities produce a surplus of energy at specific moments. The question is how can this “excess” energy be stored? One promising solution is hydrogen. Conventional hydrogen storage relies on manufactured vessels. However, scaling the technology requires larger volumes to satisfy peak demands, enhance the reliability of renewable energies, and increase hydrogen reserves for future technology and infrastructure development. The optimal solution may involve leveraging the large volumes of underground reservoirs, like salt caverns and aquifers, while minimizing the surface area usage and avoiding the manufacturing and safety issues inherent to traditional methods. There is a clear literature gap regarding the critical aspects of underground hydrogen storage (UHS) technology. Thus, a comprehensive review of the latest developments is needed to identify these gaps and guide further R&D on the topic. This work provides a better understanding of the current situation of UHS and its future challenges. It reviews the literature published on UHS, evaluates the progress in the last decades, and discusses ongoing and carried-out projects, suggesting that the technology is technically and economically ready for today’s needs.
Full article
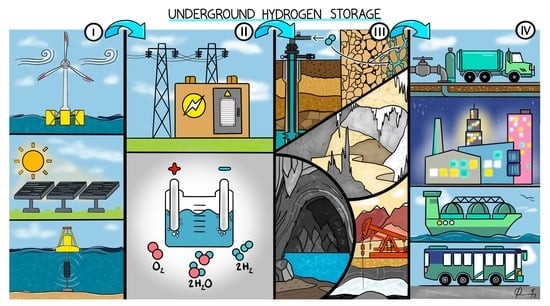
Graphical abstract
Open AccessReview
Cost Estimates and Policy Challenges of Transporting Renewable Energy Derived Ammonia from Gujarat, India to Japan
by
Takuma Otaki and Rajib Shaw
Hydrogen 2023, 4(4), 961-974; https://doi.org/10.3390/hydrogen4040056 - 25 Nov 2023
Abstract
►▼
Show Figures
With growing concern about risks related to energy security around the world, the development of hydrogen cooperation between India and Japan has become very important to ensure the economic security of the two countries and to deepen economic cooperation. This study visualizes the
[...] Read more.
With growing concern about risks related to energy security around the world, the development of hydrogen cooperation between India and Japan has become very important to ensure the economic security of the two countries and to deepen economic cooperation. This study visualizes the costs and economic issues involved in transporting Ammonia from India to Japan and discusses the policy support needed to establish a hydrogen supply chain between the two countries. If Hydrogen production is conducted in Gujarat and Ammonia production is conducted using Haber–Bosch at a large-scale Ammonia plant, the price of Ammonia at the port of Tokyo can be reduced to 572 USD/mt-NH3 if highly competitive renewable energy is utilized. For evaluating the characteristics of Ammonia produced in India, high contribution to greenhouse gas reduction, low transportation risk along transportation routes, and contribution to the diversification of energy procurement in Japan should be evaluated economically, and the following five initiatives will accelerate the composition of a Hydrogen value chain between India and Japan: (1) increasing the Indian governmental support for subsidies for Hydrogen production, (2) increasing financial support to lower capital costs, (3) ensuring a business environment to lower uncertainty about future costs, (4) promoting efforts to visualize the value of carbon credits such as JCM, and (5) visualizing the value of diversification of energy procurement sources for Japan. A graphical abstract is to follow.
Full article

Figure 1
Open AccessArticle
Cost Projection of Global Green Hydrogen Production Scenarios
by
Moe Thiri Zun and Benjamin Craig McLellan
Hydrogen 2023, 4(4), 932-960; https://doi.org/10.3390/hydrogen4040055 - 09 Nov 2023
Cited by 1
Abstract
►▼
Show Figures
A sustainable future hydrogen economy hinges on the development of green hydrogen and the shift away from grey hydrogen, but this is highly reliant on reducing production costs, which are currently too high for green hydrogen to be competitive. This study predicts the
[...] Read more.
A sustainable future hydrogen economy hinges on the development of green hydrogen and the shift away from grey hydrogen, but this is highly reliant on reducing production costs, which are currently too high for green hydrogen to be competitive. This study predicts the cost trajectory of alkaline and proton exchange membrane (PEM) electrolyzers based on ongoing research and development (R&D), scale effects, and experiential learning, consequently influencing the levelized cost of hydrogen (LCOH) projections. Electrolyzer capital costs are estimated to drop to 88 USD/kW for alkaline and 60 USD/kW for PEM under an optimistic scenario by 2050, or 388 USD/kW and 286 USD/kW, respectively, under a pessimistic scenario, with PEM potentially dominating the market. Through a combination of declining electrolyzer costs and a levelized cost of electricity (LCOE), the global LCOH of green hydrogen is projected to fall below 5 USD/kgH2 for solar, onshore, and offshore wind energy sources under both scenarios by 2030. To facilitate a quicker transition, the implementation of financial strategies such as additional revenue streams, a hydrogen/carbon credit system, and an oxygen one (a minimum retail price of 2 USD/kgO2), and regulations such as a carbon tax (minimum 100 USD/tonCO2 for 40 USD/MWh electricity), and a contract-for-difference scheme could be pivotal. These initiatives would act as financial catalysts, accelerating the transition to a greener hydrogen economy.
Full article

Figure 1
Open AccessArticle
A Computational Fluid Dynamics Analysis of Hydrogen Leakage and Nitrogen Purging of a Solid Oxide Fuel Cell Stack
by
Rasmus Dockweiler Sørensen and Torsten Berning
Hydrogen 2023, 4(4), 917-931; https://doi.org/10.3390/hydrogen4040054 - 04 Nov 2023
Abstract
A computational study of the nitrogen purging of a solid oxide fuel cell stack enclosed in a hot box is presented. The stack operates on ammonia as a fuel, and in the case of a hydrogen leakage, the entire compartment is immediately purged
[...] Read more.
A computational study of the nitrogen purging of a solid oxide fuel cell stack enclosed in a hot box is presented. The stack operates on ammonia as a fuel, and in the case of a hydrogen leakage, the entire compartment is immediately purged with nitrogen to ensure that there are no regions with high oxygen concentrations. In addition to this, the speed at which a hydrogen leak can be detected is determined. The results are then compared to a case with a relocated nitrogen inlet. A computational fluid dynamics (CFD) model is developed using the Reynolds-averaged Navier–Stokes equations for compressible flow in combination with conservation of energy and species equations in OpenFOAM. The results suggest that for the maximum concentration of oxygen to be below 5%, the hot box should be purged for 35 s, corresponding to 1.1 kg of nitrogen, if the hot box was already heated. If the hot box was at T = 300 K, it should be purged for 95 s, corresponding to 3.0 kg of nitrogen. The purge of the heated hot box results in a heat loss of 18 kW on average. A leak could be detected in 3.2 s during open circuit voltage tests. Changing the location of the outlet does not affect the cold purge, but results in a minimum purge period of 48 s during the hot purge, and the leak could be detected in 2 s. This paper demonstrates how CFD methods can be employed in order to address questions related to hydrogen safety.
Full article
(This article belongs to the Special Issue Feature Papers in Hydrogen (Volume 2))
►▼
Show Figures
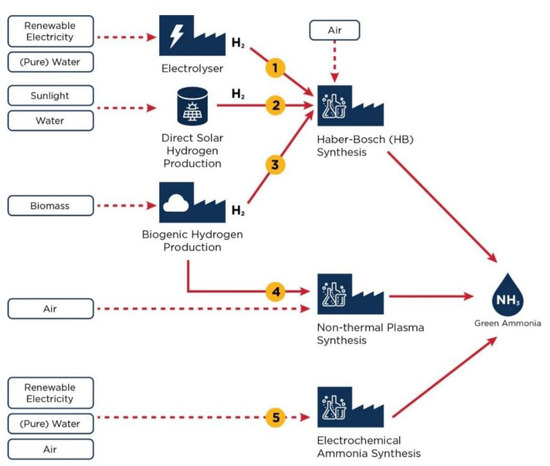
Figure 1
Open AccessReview
A Review on Mathematical Modeling of Different Biological Methods of Hydrogen Production
by
Priyakrishna Yumnam and Pradip Debnath
Hydrogen 2023, 4(4), 881-916; https://doi.org/10.3390/hydrogen4040053 - 01 Nov 2023
Abstract
►▼
Show Figures
In this paper, we present an updated review on the mathematical modeling of different biological methods of hydrogen production. The presented mathematical modeling and methods range from inception to the current state-of-the-art developments in hydrogen production using biological methods. A comparative study was
[...] Read more.
In this paper, we present an updated review on the mathematical modeling of different biological methods of hydrogen production. The presented mathematical modeling and methods range from inception to the current state-of-the-art developments in hydrogen production using biological methods. A comparative study was performed along with indications for future research and shortcomings of earlier research. This review will be helpful for all researchers working on different methods of hydrogen production. However, we only covered biological methods such as biophotolysis, fermentation and microbial electrolysis cells, and this list is not exhaustive of all other methods of hydrogen production.
Full article
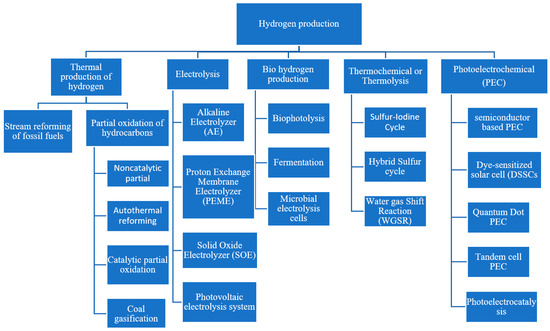
Figure 1
Open AccessArticle
Thermodynamics of Reversible Hydrogen Storage: Are Methoxy-Substituted Biphenyls Better through Oxygen Functionality?
by
Sergey P. Verevkin, Artemiy A. Samarov and Sergey V. Vostrikov
Hydrogen 2023, 4(4), 862-880; https://doi.org/10.3390/hydrogen4040052 - 20 Oct 2023
Abstract
The reversible hydrogenation/dehydrogenation of aromatic molecules, known as liquid organic hydrogen carriers, is considered as an attractive option for the safe storage and release of elemental hydrogen. The recently reported efficient synthetic routes to obtain methoxy-biphenyls in high yield make them promising candidates
[...] Read more.
The reversible hydrogenation/dehydrogenation of aromatic molecules, known as liquid organic hydrogen carriers, is considered as an attractive option for the safe storage and release of elemental hydrogen. The recently reported efficient synthetic routes to obtain methoxy-biphenyls in high yield make them promising candidates for hydrogen storage. In this work, a series of methoxy-substituted biphenyls and their structural parent compounds were studied. The absolute vapour pressures were measured using the transpiration method and the enthalpies of vaporisation/sublimation were determined. We applied a step-by-step procedure including structure–property correlations and quantum chemical calculations to evaluate the quality of thermochemical data on the enthalpies of phase transitions and enthalpies of formation of the studied methoxy compounds. The data sets on thermodynamic properties were evaluated and recommended for calculations in chemical engineering. A thermodynamic analysis of chemical reactions based on methoxy-biphenyls in the context of hydrogen storage was carried out and the energetics of these reactions were compared with the energetics of reactions of common LOHCs. The influence of the position of the methoxy groups in the rings on the enthalpies of the reactions relevant for hydrogen storage was discussed.
Full article
(This article belongs to the Special Issue Feature Papers in Hydrogen (Volume 2))
►▼
Show Figures
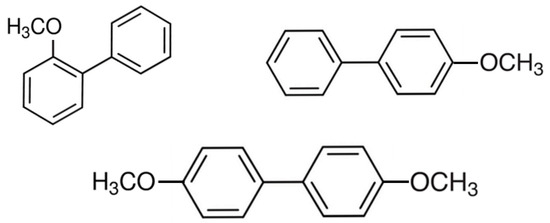
Figure 1
Open AccessReview
Hydrogen Storage as a Key Energy Vector for Car Transportation: A Tutorial Review
by
Marie-Charlotte Dragassi, Laurent Royon, Michaël Redolfi and Souad Ammar
Hydrogen 2023, 4(4), 831-861; https://doi.org/10.3390/hydrogen4040051 - 19 Oct 2023
Cited by 1
Abstract
►▼
Show Figures
Hydrogen storage is a key enabling technology for the extensive use of hydrogen as energy carrier. This is particularly true in the widespread introduction of hydrogen in car transportation. Indeed, one of the greatest technological barriers for such development is an efficient and
[...] Read more.
Hydrogen storage is a key enabling technology for the extensive use of hydrogen as energy carrier. This is particularly true in the widespread introduction of hydrogen in car transportation. Indeed, one of the greatest technological barriers for such development is an efficient and safe storage method. So, in this tutorial review the existing hydrogen storage technologies are described with a special emphasis on hydrogen storage in hydrogen cars: the current and the ongoing solutions. A particular focus is given on solid storage and some of the recent advances on plasma hydrogen ion implantation, which should allow not only the preparation of metal hydrides, but also the imagination of a new refluing circuit. From hydrogen discovery to its use as an energy vector in cars, this review wants to be as exhaustive as possible, introducing the basics of hydrogen storage, and discussing the experimental practicalities of car hydrogen fuel. It wants to serve as a guide for anyone wanting to undertake such a technology and to equip the reader with an advanced knowledge on hydrogen storage and hydrogen storage in hydrogen cars to stimulate further researches and yet more innovative applications for this highly interesting field.
Full article
Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Batteries, Catalysts, Energies, Hydrogen, Sustainability
Preparation, Storage, and Transportation of Green Hydrogen and Multi-Scenario Application Technologies
Topic Editors: Weihua Cai, Chao Xu, Zhonghao Rao, Fuqiang Wang, Ming GaoDeadline: 30 June 2024
Topic in
Energies, Materials, Catalysts, Metals, Hydrogen
Hydrogen—The New Energy Vector for the Transition of Industries "Hard to Abate"
Topic Editors: Pasquale Cavaliere, Geoffrey BrooksDeadline: 31 August 2024
Topic in
Catalysts, Hydrogen, Molecules, Nanomaterials, Physchem
Fabrication of Hybrid Materials for Catalysis
Topic Editors: Jerry J. Wu, Michael Arkas, Dimitrios GiannakoudakisDeadline: 30 September 2024
Topic in
Energies, Sustainability, Batteries, Clean Technol., Hydrogen
Hydrogen Technologies vs. Battery Ones in the Green Energy Transition
Topic Editors: Orazio Barbera, Monica Santamaria, Vincenzo BaglioDeadline: 20 November 2024

Conferences
Special Issues
Special Issue in
Hydrogen
Promising Electrocatalysts for Hydrogen Production in Acidic Environment
Guest Editor: Mohammed-Ibrahim JameshDeadline: 30 September 2024
Special Issue in
Hydrogen
Recent Advances in Hydrogen Technologies: Production, Storage and Utilization
Guest Editors: Rajender Boddula, Lakshmana Reddy NagappagariDeadline: 31 October 2024