Influence of Agricultural Expansion and Human Disturbance on the Encounter Rates of Nocturnal Mammals in Tropical Hill Forests in Bangladesh
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Survey Methods
2.3. Analysis
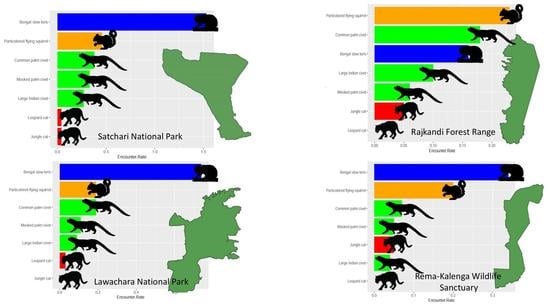
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pillay, R.; Venter, M.; Aragon-Osejo, J.; González-del-Pliego, P.; Hansen, A.J.; Watson, J.E.; Venter, O. Tropical forests are home to over half of the world’s vertebrate species. Front. Ecol. Environ. 2022, 20, 10–15. [Google Scholar] [CrossRef]
- Vira, B.; Wildburger, C.; Mansourian, M. Forests, Trees and Landscapes for Food Security and Nutrition. In A Global Assessment Report; IUFRO: Vienna, Austria, 2015. [Google Scholar]
- Scholes, R.J.; Walters, M.; Turak, E.; Saarenmaa, H.; Heip, C.H.; Tuama, É.Ó.; Faith, D.P.; Mooney, H.A.; Ferrier, S.; Jongman, R.H. Building a global observing system for biodiversity. Curr. Opin. Environ. Sustain. 2012, 4, 139–146. [Google Scholar] [CrossRef]
- Velastegui-Montoya, A.; Montalván-Burbano, N.; Peña-Villacreses, G.; de Lima, A.; Herrera-Franco, G. Land use and land cover in tropical forest: Global research. Forests 2022, 13, 1709. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, P.G.; Slay, C.M.; Harris, N.L.; Tyukavina, A.; Hansen, M.C. Classifying drivers of global forest loss. Science 2018, 361, 1108–1111. [Google Scholar] [CrossRef] [PubMed]
- Laurance, W.F.; Sayer, J.; Cassman, K.G. Agricultural expansion and its impacts on tropical nature. Trends Ecol. Evol. 2014, 29, 107–116. [Google Scholar] [CrossRef]
- Knoke, T.; Gosling, E.; Reith, E.; Gerique, A.; Pohle, P.; Valle Carrión, L.; Ochoa Moreno, W.S.; Castro, L.M.; Calvas, B.; Hildebrandt, P.; et al. Confronting sustainable intensification with uncertainty and extreme values on smallholder tropical farms. Sustain. Sci. 2022, 17, 1977–1994. [Google Scholar] [CrossRef]
- Sher, A.A.; Marshall, D.L.; Gilbert, S.A. Competition between native Populus deltoides and invasive Tamarix ramosissima and the implications for reestablishing flooding disturbance. Conserv. Biol. 2000, 14, 1744–1754. [Google Scholar] [CrossRef]
- Sousa, W.P. The role of disturbance in natural communities. An. Rev. Ecol. Syst. 1984, 15, 353–391. [Google Scholar] [CrossRef]
- Ni, Q.; Yu, G.; Nijman, V.; Nekaris, K.A.I.; Xu, H.; Zhang, M.; Yao, Y.; Xie, M. Spatial heterogeneity and socioeconomic transformation challenge the prevention of illegal wildlife consumption in China. Biol. Cons. 2022, 275, 109751. [Google Scholar] [CrossRef]
- Chaves, L.S.M.; Fry, J.; Malik, A.; Geschke, A.; Sallum, M.A.M.; Lenzen, M. Global consumption and international trade in deforestation-associated commodities could influence malaria risk. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franquesa-Soler, M.; Aristizabal, J.F.; Andresen, E.; del Burgo, I.V.; Shedden-González, A.; Rodríguez-Luna, E. Long-term assessment of the translocation of an endangered primate into an agroforestry system. Endanger. Species Res. 2022, 48, 235–246. [Google Scholar] [CrossRef]
- Estrada, A.; Raboy, B.E.; Oliveira, L.C. Agroecosystems and primate conservation in the tropics: A review. Am. J. Primatol. 2012, 74, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.T.M.; Bernardo, C.S.S.; De Melo, F.R.; Santos-Filho, M.D.; Peres, C.A.; Canale, G.R. Primate and ungulate responses to teak agroforestry in a southern Amazonian landscape. Mammal. Biol. 2019, 96, 45–52. [Google Scholar] [CrossRef]
- Imron, M.A.; Campera, M.; Al Bihad, D.; Rachmawati, F.D.; Nugroho, F.E.; Budiadi, B.; Wianti, K.F.; Suprapto, E.; Nijman, V.; Nekaris, K.A.I. Bird assemblages in coffee agroforestry systems and other human modified habitats in Indonesia. Biology 2022, 11, 310. [Google Scholar] [CrossRef]
- Campera, M.; Hedger, K.; Birot, H.; Manson, S.; Balestri, M.; Budiadi, B.; Imron, M.A.; Nijman, V.; Nekaris, K.A.I. Does the presence of shade trees and distance to the forest affect detection rates of terrestrial vertebrates in coffee home gardens? Sustainability 2021, 13, 8540. [Google Scholar] [CrossRef]
- The Food and Agriculture Organization of the United Nations. Forest Resources of Bangladesh Country Report; Food and Agricultural Organisation: Rome, Italy, 2020. [Google Scholar]
- Iftekhar, M.S.; Saenger, P. Vegetation dynamics in the Bangladesh Sundarbans mangroves: A review of forest inventories. Wetlands Ecol. Manag. 2008, 16, 291–312. [Google Scholar] [CrossRef]
- Loucks, C.; Barber-Meyer, S.; Hossain, M.A.A.; Barlow, A.; Chowdhury, R.M. Sea level rise and tigers: Predicted impacts to Bangladesh’s Sundarbans mangroves. Clim. Chang. 2010, 98, 291. [Google Scholar] [CrossRef]
- Barlow, A.C.; Ahmed, M.I.U.; Rahman, M.M.; Howlader, A.; Smith, A.C.; Smith, J.L. Linking monitoring and intervention for improved management of tigers in the Sundarbans of Bangladesh. Biol. Conserv. 2008, 141, 2032–2040. [Google Scholar] [CrossRef]
- Gopal, B.; Chauhan, M. Biodiversity and its conservation in the Sundarban mangrove ecosystem. Aquat. Sci. 2006, 68, 338–354. [Google Scholar] [CrossRef]
- Champion, S.H.; Seth, S.K.; Khattak, G.M. Forest Types of Pakistan; Pakistan Forest Institute: Peshawar, Pakistan, 1965.
- Falchi, F.; Cinzano, P.; Duriscoe, D.; Kyba, C.C.; Elvidge, C.D.; Baugh, K.; Portnov, B.A.; Rybnikova, N.A.; Furgoni, R. The new world atlas of artificial night sky brightness. Sci. Adv. 2016, 2, e1600377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewers, R.M.; Didham, R.K. Confounding factors in the detection of species responses to habitat fragmentation. Biol. Rev. 2006, 81, 117–142. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.A.; Willis, K.J.; Birks, H.J.B.; Whittaker, R.J. Agroforestry: A refuge for tropical biodiversity? Trends Ecol. Evol. 2008, 23, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, J.K.; Biswas, S.R.; Islam, S.M.; Rahman, O.; Uddin, S.N. Biodiversity of Satchari Reserved Forest, Habiganj; IUCN Bangladesh Country Office: Dhaka, Bangladesh, 2004. [Google Scholar]
- Mukul, S.A.; Rashid, A.Z.M.M.; Quazi, S.A.; Uddin, M.B.; Fox, J. Local peoples’ responses to co-management regime in protected areas: A case study from Satchari National Park, Bangladesh. For. Trees Livelihoods 2012, 21, 16–29. [Google Scholar] [CrossRef]
- Quazi, S.A.; Ticktin, T. Understanding drivers of forest diversity and structure in managed landscapes: Secondary forests, plantations, and agroforests in Bangladesh. For. Ecol. Manag. 2016, 366, 118–134. [Google Scholar] [CrossRef]
- Rahman, H.; Khurshed, A. Forest dependent indigenous communities’ perception and adaptation to climate change through local knowledge in the Protected Area—A Bangladesh case study. Climate 2016, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.Z.; Roy, S. Collection and Management of Selected Medicinal Plants in Rema-Kalenga Wildlife Sanctuary. In Making Conservation Work: Linking Rural Livelihoods and Protected Area Management in Bangladesh; Fox, J., Bushley, B.R., Dutt, S., Quazi, S.A., Eds.; East–West Center and Nishorgo Program, Bangladesh Forest Department: Dhaka, Bangladesh, 2007; pp. 66–83. [Google Scholar]
- Rahman, M.H.; Miah, M.D. Are protected forests of Bangladesh prepared for the implementation of REDD+? A forest governance analysis from Rema-Kalenga Wildlife Sanctuary. Environments 2017, 4, 43. [Google Scholar] [CrossRef] [Green Version]
- Walsh, P.D.; White, L.J.T. What will it take to monitor forest elephants? Conserv. Biol. 1999, 13, 1194–1202. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R.; Laake, J.L. Estimation of density from line transect sampling of biological populations. Wildl. Monogr. 1980, 72, 3–202. [Google Scholar]
- Das, N.; Nekaris, K.A.I.; Bhattacharjee, P.C. Medicinal plant exudativory by the Bengal slow loris Nycticebus bengalensis. Endanger. Species Res. 2014, 23, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Al-Razi, H.; Maria, M. Population status of particolored flying squirrel (Hylopetes alboniger) in four forest patches of northeast Bangladesh. NeBIO 2019, 10, 77–79. [Google Scholar]
- Nekaris, K.A.I.; Jayewardene, J. Pilot study and conservation status of the slender loris (Loris tardigradus and L. lydekkerianus) in Sri Lanka. Prim. Cons. 2003, 19, 83–90. [Google Scholar]
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.R.; Beher, J.; Jones, K.R.; Possingham, H.P.; Laurence, W.F.; Wood, P.; Fekete, B.M.; et al. Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nat. Comm. 2016, 7, 12558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wildlife Conservation Society-WCS, and Center for International Earth Science Information Network-CIESIN-Columbia University. Last of the Wild Project, Version 2, 2005 (LWP-2): Global Human Influence Index (HII) Dataset (Geographic); NASA Socioeconomic Data and Applications Center (SEDAC): Palisades, NY, USA, 2005. [Google Scholar] [CrossRef]
- Benítez-López, A.; Alkemade, R.; Schipper, A.M.; Ingram, D.J.; Verweij, P.A.; Eikelboom, J.A.J.; Huijbregts, M.A.J. The impact of hunting on tropical mammal and bird populations. Science 2017, 356, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gittins, S.P.; Akonda, A.W. What survives in Bangladesh? Oryx 1982, 16, 275–282. [Google Scholar] [CrossRef]
- Khan, M.A.R. Endangered mammals of Bangladesh. Oryx 1984, 18, 152–156. [Google Scholar] [CrossRef]
- Sarker, S.U.; Sarker, N.J. Mammals of Bangladesh—Their status, distribution and habitat. Tigerpaper 1984, 11, 8–12. [Google Scholar]
- Aziz, M.A. Notes on the status of mammalian fauna of the Lawachara National Park, Bangladesh. Ecoprint Int. J. Ecol. 2011, 18, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Nekaris, K.A.I.; Nijman, V. CITES proposal highlights rarity of Asian nocturnal primates (Lorisidae: Nycticebus). Folia Primatol. 2007, 78, 211. [Google Scholar] [CrossRef]
- Nekaris, K.A.I.; Blackham, G.V.; Nijman, V. Conservation implications of low encounter rates of five nocturnal primate species (Nycticebus spp.) in Asia. Biodiv. Conserv. 2008, 17, 733–747. [Google Scholar] [CrossRef]
- Nekaris, K.A.I.; Pambudi, J.A.A.; Susanto, D.; Ahmad, R.D.; Nijman, V. Densities, distribution and detectability of a small nocturnal primate (Javan slow loris Nycticebus javanicus) in a montane rainforest. Endanger. Species. Res. 2014, 24, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Thorn, J.S.; Nijman, V.; Smith, D.; Nekaris, K.A.I. Ecological niche modelling as a technique for assessing threats and setting conservation priorities for Asian slow lorises (Primates: Nycticebus). Diver. Distr. 2009, 1, 289–298. [Google Scholar] [CrossRef]
- Al-Razi, H.; Maria, M.; Muzaffar, S.B. Mortality of primates due to roads and power lines in two forest patches in Bangladesh. Zoologia 2019, 36, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Nekaris, K.A.I.; Starr, C.R. Conservation and ecology of the neglected slow loris: Priorities and prospects. Endanger. Species Res. 2015, 28, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Murali Krishna, C.; Ray, P.C.; Sarma, K.; Kumar, A. Observations on particoloured flying squirrel Hylopetes aloniger in Northeast India. Zoos Print 2013, 18, 18–20. [Google Scholar]
- Adhikarimayum, A.S.; Hazra, P.; Mikhu, J. Conservation status and threats of lesser known Sciuridae species in Dibang Valley District of Arunachal Pradesh, India. Int. Res. J. Biol. Sci. 2021, 10, 25–31. [Google Scholar]
- Moore, J.H.; Sittimongkol, S.; Campos-Arceiz, A.; Sumpah, T.; Eichhorn, M.P. Fruit gardens enhance mammal diversity and biomass in a southeast Asian rainforest. Biol. Conserv. 2016, 194, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Rode-Margono, E.J.; Voskamp, A.; Spaan, D.; Lehtinen, J.K.; Roberts, P.D.; Nijman, V.; Nekaris, K.A.I. Records of small carnivores and of medium-sized nocturnal mammals on Java, Indonesia. Small Carniv. Conserv. 2014, 50, 1–11. [Google Scholar]
- Iseborn, T.; Rogers, L.D.; Rawson, B.; Nekaris, K.A.I. Sightings of Common Palm Civets Paradoxurus hermaphroditus and of other civet species at Phnom Samkos Wildlife Sanctuary and Veun Sai–Siem Pang Conservation Area, Cambodia. Small Carniv. Conserv. 2012, 46, 26–29. [Google Scholar]
- Krishna, C.M.; Kumar, A.; Ray, P.C.; Sarma, K.; Deka, J. Investigating the Foraging Patterns and Distribution of Nocturnal Frugivores with Special Focus on Conservation Threats in Namdapha National Park, Arunachal Pradesh, India; Rufford Small Grants Program: London, UK, 2015. [Google Scholar]
- Radhakrishna, S.; Datta-Roy, A.; Swapna, N.; Sinha, A. Population survey of the Bengal slow loris, Nycticebus bengalensis, in Meghalaya, Northeast India. Primate Conserv. 2010, 25, 105–111. [Google Scholar] [CrossRef]
- Radhakrishna, S.; Sinha, A. Population Survey and Conservation of the Bengal Slow Loris in Assam and Meghalaya, North-Eastern India; National Institute of Advanced Studies: Bangalore, Indian, 2004. [Google Scholar]
- Das, N.; Nekaris, K.A.I.; Biswas, J.; Das, J.; Bhattacharjee, P.C. Persistence and protection of the Vulnerable Bengal Slow Loris Nycticebus bengalensis in Assam and Arunachal Pradesh, north-east India. Oryx 2015, 49, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Das, N.; Biswas, J.; Bhatacharya, K.; Nekaris, K.A.I. Observatons on the Bengal Slow Loris Nyctcebus bengalensis in Pakke Tiger Reserve, Arunachal Pradesh, India. Asian Primates J. 2016, 6, 27–32. [Google Scholar]
- Duckworth, J.W. Field sightings of the pygmy Loris, Nycticebus pygmaeus in Laos. Folia Primatol. 1994, 63, 99–101. [Google Scholar] [CrossRef]
- Evans, T.D.; Piseth, H.; Phaktra, P.; Mary, H. A Study of Resin-Tapping and Livelihoods in Southern Mondulkiri, Cambodia, with Implications for Conservation and Forest Management; Wildlife Conservation Society–Cambodia Program: Phnom Penh, Cambodia, 2003. [Google Scholar]
- Pliosungnoen, M.; Gale, G.; Savini, T. Density and microhabitat use of Bengal slow loris in primary forest and non-native plantation forest. Am. J. Primatol. 2010, 72, 1108–1117. [Google Scholar] [CrossRef]
- Coudrat, C.; Rogers, L.; Nekaris, K. Abundance of primates reveals Samkos Wildlife Sanctuary, Cardamom Mountains, Cambodia as a priority area for conservation. Oryx 2011, 45, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Gregory, T.; Carrasco Rueda, F.; Deichmann, J.; Kolowski, J.; Alonso, A. Arboreal camera trapping: Taking a proven method to new heights. Methods Ecol. Evol. 2014, 5, 443–451. [Google Scholar] [CrossRef]
- Yihao, F.; Guopeng, R.; Ying, G.; Shuxia, Z.; Haohan, W.; Yanpeng, L.; Zhipang, H.; Liangwei, C.; Wen, X. Impact of ground and canopy camera-trapping installation on wildlife monitoring. Biodivers. Sci. 2018, 26, 717. [Google Scholar]
- Kumara, H.N.; Singh, M. Small carnivores of Karnataka: Distribution and sight records. J. Bombay Nat. Hist. Soc. 2007, 104, 155–162. [Google Scholar]
- Noor, A.; Ahmed, K.; Mir, Z.R. Saleem-ul-Haq Estimating abundance of some wild faunal elements of Jasrota Wildlife Sanctuary, India. J. King Saud. Univ. Sci. 2016, 28, 232–238. [Google Scholar] [CrossRef] [Green Version]
- Srivathsa, A.; Parameshwaran, R.; Sharma, S.; Karanth, K.U. Estimating population sizes of leopard cats in the Western Ghats using camera surveys. J. Mamm. 2015, 96, 742–750. [Google Scholar] [CrossRef]
- Kalle, R.; Ramesh, T.; Qureshi, Q.; Sankar, K. Estimating seasonal abundance and habitat use of small carnivores in the Western Ghats using an occupancy approach. J. Tropic. Ecol. 2014, 30, 469–480. [Google Scholar] [CrossRef]
- Sathyakumar, S.; Bashir, T.; Bhattacharya, T.; Poudyal, K. Assessing mammal distribution and abundance in intricate eastern Himalayan habitats of Khangchendzonga, Sikkim, India. Mammalia 2011, 75, 257–268. [Google Scholar] [CrossRef]
- Jennings, A.P.; Naim, M.; Advento, A.D.; Aryawan, A.A.K.; Ps, S.; Caliman, J.P.; Verwilghen, A.; Veron, G. Diversity and occupancy of small carnivores within oil palm plantations in central Sumatra, Indonesia. Mammal Res. 2015, 60, 181–188. [Google Scholar] [CrossRef]
- Bali, A.; Kumar, A.; Krishnaswamy, J. The mammalian communities in coffee plantations around a protected area in the Western Ghats, India. Biol. Conserv. 2007, 139, 93–102. [Google Scholar] [CrossRef]
- Azhar, B.; Lindenmayer, D.B.; Wood, J.; Fischer, J.; Zakaria, M. Ecological impacts of oil palm agriculture on forest mammals in plantation estates and smallholdings. Biodivers. Conserv. 2014, 23, 1175–1191. [Google Scholar] [CrossRef]
- Gaston, K.J. Nighttime Ecology: The “Nocturnal Problem” Revisited. Am. Naturalist 2019, 193, 481–502. [Google Scholar] [CrossRef] [PubMed]
- Park, O. Nocturnalism—The development of a problem. Ecol. Monographs 1940, 10, 485–536. [Google Scholar] [CrossRef]
- Hölker, F.; Wolter, C.; Perkin, E.K.; Tockner, K. Light pollution as a biodiversity threat. Trends Ecol. Evol. 2010, 25, 681–682. [Google Scholar] [CrossRef]
- Reza, A.A.; Hasan, M.K. Forest biodiversity and deforestation in Bangladesh: The latest update. In Forest Degradation around the World; Intech Open: London, UK, 2020; pp. 1–19. [Google Scholar]
- Aziz, A.; Barlow, A.C.; Greenwood, C.C.; Islam, A. Prioritizing threats to improve conservation strategy for the tiger Panthera tigris in the Sundarbans Reserve Forest of Bangladesh. Oryx 2013, 47, 510–518. [Google Scholar] [CrossRef] [Green Version]
- Harvey, C.A.; González Villalobos, J.A. Agroforestry systems conserve species-rich but modified assemblages of tropical birds and bats. Biodivers. Conserv. 2007, 16, 2257–2292. [Google Scholar] [CrossRef]


| Site | Total Transect Length (km) | Monoculture with Semi-Natural Forest (km) | Semi-Natural Forest (km) | Gardens with Semi-Natural Forest (km) | Human Influence Index |
|---|---|---|---|---|---|
| Satchari | 21.54 | 7.92 | 4.92 | 8.70 | 18.0 |
| Lawachara | 36.05 | 10.10 | 14.32 | 11.63 | 22.0 |
| Rajkandi | 36.86 | 3.64 | 28.74 | 4.48 | 10.2 |
| Rema-Kalenga | 24.46 | 0.00 | 22.35 | 2.11 | 14.6 |
| Species | Satchari (n = 13) | Lawachara (n = 18) | Rajkandi (n = 15) | Rema-Kalenga (n = 9) |
|---|---|---|---|---|
| Bengal slow loris | 1.53 (0.16) a | 0.74 (0.11) b | 0.16 (0.06) c | 0.34 (0.12) c |
| Particolored flying squirrel | 0.46 (0.14) | 0.19 (0.07) | 0.23 (0.09) | 0.20 (0.09) |
| Common palm civet | 0.38 (0.11) a | 0.19 (0.07) | 0.18 (0.08) | 0.07 (0.05) b |
| Masked palm civet | 0.33 (0.10) a | 0.11 (0.07) | 0.06 (0.04) b | 0.05 (0.05) b |
| Large Indian civet | 0.27 (0.12) a | 0.09 (0.05) | 0.10 (0.07) | 0.04 (0.04) b |
| Jungle cat | 0.04 (0.04) | 0.00 (0.00) | 0.05 (0.03) | 0.05 (0.05) |
| Leopard cat | 0.04 (0.04) | 0.03 (0.03) | 0.00 (0.00) | 0.00 (0.00) |
| Response Variable | Predictor a | Estimate | Std. Error | Z Value | p Value |
|---|---|---|---|---|---|
| Bengal slow loris | Intercept | −2.71 | 1.12 | −2.42 * | 0.016 |
| Habitat: Garden | −0.16 | 0.30 | −0.53 | 0.599 | |
| Habitat: Monoculture | −0.02 | 0.31 | −0.07 | 0.944 | |
| HII | 0.13 | 0.06 | 1.97 * | 0.048 | |
| Common palm civet | Intercept | −1.93 | 0.77 | −2.51 * | 0.012 |
| Habitat: Garden | 0.86 | 0.52 | 1.68 | 0.093 | |
| Habitat: Monoculture | 0.41 | 0.64 | 0.63 | 0.527 | |
| HII | −0.01 | 0.05 | −0.18 | 0.861 | |
| Large Indian civet | Intercept | −3.24 | 1.35 | −2.41 * | 0.016 |
| Habitat: Garden | 2.40 | 0.84 | 2.85 ** | 0.004 | |
| Habitat: Monoculture | 1.23 | 1.05 | 1.17 | 0.241 | |
| HII | −0.01 | 0.07 | −0.19 | 0.850 | |
| Masked palm civet | Intercept | −4.45 | 1.84 | −2.41 * | 0.016 |
| Habitat: Garden | 0.80 | 0.67 | 1.21 | 0.228 | |
| Habitat: Monoculture | 0.10 | 0.81 | 0.12 | 0.901 | |
| HII | 0.12 | 0.11 | 1.10 | 0.273 | |
| Particolored flying squirrel | Intercept | −1.62 | 0.68 | −2.39 * | 0.017 |
| Habitat: Garden | 0.93 | 0.47 | 1.99 * | 0.047 | |
| Habitat: Monoculture | 0.65 | 0.54 | 1.19 | 0.235 | |
| HII | −0.02 | 0.04 | −0.39 | 0.698 |
| Species | Encounter Rate | Location | Habitat Type | References |
|---|---|---|---|---|
| Bengal Slow Loris | 0.04–0.10 | Meghalaya, India | Forest | [57] |
| 0.05–0.33 | Assam, India | Forest | [58,59] | |
| 0.20–0.26 | Arunachal Pradesh, India | Forest | [59,60] | |
| 0.04–0.65 | Central Laos | Forest | [61,62] | |
| 0.40–0.87 | South Laos | Forest | [62] | |
| 0.34 | Thailand | Forest | [63] | |
| 0.45–1.02 | Thailand | Garden | [63] | |
| 0.38–0.50 | Cambodia | Forest | [64] | |
| 0.47 | Bangladesh | Forest | This study | |
| 0.78 | Bangladesh | Garden | This study | |
| 0.97 | Bangladesh | Monoculture | This study | |
| Common palm civet | 0.35–0.39 | Cambodia | Forest | [55] |
| 0.34 | Java, Indonesia | Forest | [54] | |
| 0.18 | Java, Indonesia | Garden | [54] | |
| 0.20 | Arunachal Pradesh, India | Forest | [56] | |
| 0.00–0.10 | Karnakata, India | Forest | [65] | |
| 0.13 | Bangladesh | Forest | This study | |
| 0.30 | Bangladesh | Garden | This study | |
| 0.18 | Bangladesh | Monoculture | This study | |
| Masked Palm Civet | 0.20 | Arunachal Pradesh, India | Forest | [56] |
| 0.07 | Bangladesh | Forest | This study | |
| 0.26 | Bangladesh | Garden | This study | |
| 0.14 | Bangladesh | Monoculture | This study | |
| Large Indian Civet | 0.03 | Bangladesh | Forest | This study |
| 0.30 | Bangladesh | Garden | This study | |
| 0.09 | Bangladesh | Monoculture | This study | |
| Jungle cat | 0.00–0.05 | Karnakata, India | Forest | [65] |
| 0.03 | Jammu and Kashmir, India | Forest | [66] | |
| 0.06 | Bangladesh | Forest | This study | |
| 0.00 | Bangladesh | Garden | This study | |
| 0.00 | Bangladesh | Monoculture | This study | |
| Leopard cat | 0.00–0.07 | Karnataka, India | Forest | [65,67] |
| 0.00 | Bangladesh | Forest | This study | |
| 0.07 | Bangladesh | Garden | This study | |
| 0.00 | Bangladesh | Monoculture | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Razi, H.; Campera, M.; Hasan, S.; Maria, M.; Nijman, V.; Nekaris, K.A.-I. Influence of Agricultural Expansion and Human Disturbance on the Encounter Rates of Nocturnal Mammals in Tropical Hill Forests in Bangladesh. Ecologies 2023, 4, 195-208. https://doi.org/10.3390/ecologies4010014
Al-Razi H, Campera M, Hasan S, Maria M, Nijman V, Nekaris KA-I. Influence of Agricultural Expansion and Human Disturbance on the Encounter Rates of Nocturnal Mammals in Tropical Hill Forests in Bangladesh. Ecologies. 2023; 4(1):195-208. https://doi.org/10.3390/ecologies4010014
Chicago/Turabian StyleAl-Razi, Hassan, Marco Campera, Sabit Hasan, Marjan Maria, Vincent Nijman, and K. Anne-Isola Nekaris. 2023. "Influence of Agricultural Expansion and Human Disturbance on the Encounter Rates of Nocturnal Mammals in Tropical Hill Forests in Bangladesh" Ecologies 4, no. 1: 195-208. https://doi.org/10.3390/ecologies4010014