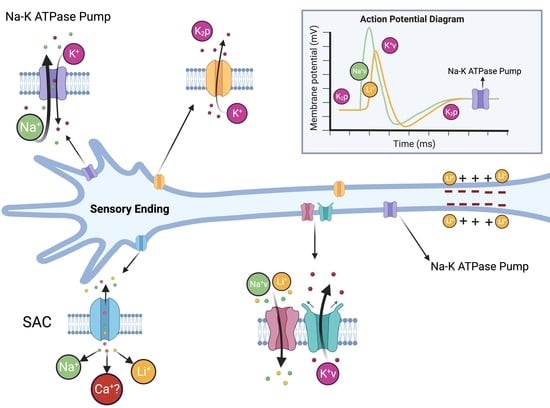
The Effects of Lithium on Proprioceptive Sensory Function and Nerve Conduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Dissection and Physiology
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janka, Z.; Jones, D.G. Lithium entry into neural cells via sodium channels: A morphometric approach. Neuroscience 1982, 7, 2849–2857. [Google Scholar] [CrossRef]
- Obara, S.; Grundfest, H. Effects of lithium on different membrane components of crayfish stretch receptor neurons. J. Gen. Physiol. 1968, 51, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Baldessarini, R.J.; Tondo, L.; Vázquez, G.H. Pharmacological treatment of adult bipolar disorder. Mol. Psychiatry 2019, 24, 198–217. [Google Scholar] [CrossRef] [PubMed]
- Fountoulakis, K.N.; Tohen, M.; Zarate, C.A., Jr. Lithium treatment of Bipolar disorder in adults: A systematic review of randomized trials and meta-analyses. Eur. Neuropsychopharmacol. 2022, 54, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Baird-Gunning, J.; Lea-Henry, T.; Hoegberg, L.C.G.; Gosselin, S.; Roberts, D.M. Lithium poisoning. J. Intensive Care Med. 2017, 32, 249–263. [Google Scholar] [CrossRef]
- Mifsud, S.; Cilia, K.; Mifsud, E.L.; Gruppetta, M. Lithium-associated hyperparathyroidism. Br. J. Hosp. Med. 2020, 81, 1–9. [Google Scholar] [CrossRef]
- Kato, M.; Lledo, P.M.; Vincent, J.D. Blockade by lithium ions of potassium channels in rat anterior pituitary cells. Am. J. Physiol. 1991, 261 Pt 1, C218–C223. [Google Scholar] [CrossRef] [PubMed]
- Carrasquillo, Y.; Nerbonne, J.M. IA channels: Diverse regulatory mechanisms. Neuroscientist 2014, 20, 104–111. [Google Scholar] [CrossRef]
- Thomas, L.; Xue, J.; Dominguez Rieg, J.A.; Rieg, T. Contribution of NHE3 and dietary phosphate to lithium pharmacokinetics. Eur. J. Pharm. Sci. 2019, 128, 1–7. [Google Scholar] [CrossRef]
- Banerjee, U.; Dasgupta, A.; Rout, J.K.; Singh, O.P. Effects of lithium therapy on Na+-K+-ATPase activity and lipid peroxidation in bipolar disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 37, 56–61. [Google Scholar] [CrossRef]
- Fleĭshman, D.G. Li+ as a Na+ analog in ion transport process in vertebrates. Tsitologiia 1991, 33, 111–117. (In Russian) [Google Scholar] [PubMed]
- Dudev, T.; Mazmanian, K.; Lim, C. Competition between Li+ and Na+ in sodium transporters and receptors: Which Na+-Binding sites are “therapeutic” Li+ targets? Chem. Sci. 2018, 9, 4093–4103. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.L.; Silva Júnior, G.B.; Abreu, K.L.; Rocha Nde, A.; Franco, L.F.; Araújo, S.M.; Daher Ede, F. Lithium nephrotoxicity. Rev. Assoc. Med. Bras. 2010, 56, 600–606, (In English, In Portuguese). [Google Scholar] [CrossRef]
- Gitlin, M. Lithium side effects and toxicity: Prevalence and management strategies. Int. J. Bipolar Disord. 2016, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Berk, M. The putative use of lithium in Alzheimer’s disease. Curr. Alzheimer Res. 2016, 13, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Nyirenda, M.J.; Tang, J.I.; Padfield, P.L.; Seckl, J.R. Hyperkalaemia. Brit. Med. J. 2009, 339, b4114. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Horowicz, P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J. Physiol. 1959, 148, 127–160. [Google Scholar] [CrossRef]
- Kristensen, S.R. Mechanisms of cell damage and enzyme release. Danish Med. Bull. 1994, 41, 423–433. [Google Scholar]
- Orkand, R.K.; Nicholls, J.G.; Kuffler, S.W. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J. Neurophysiol. 1966, 29, 788–806. [Google Scholar] [CrossRef]
- Grafe, P.; Reddy, M.M.; Emmert, H.; ten Bruggencate, G. Effects of lithium on electrical activity and potassium ion distribution in the vertebrate central nervous system. Brain Res. 1983, 279, 65–76. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- Arnadóttir, J.; Chalfie, M. Eukaryotic mechanosensitive channels. Annu. Rev. Biophys. 2010, 39, 111–137. [Google Scholar] [CrossRef]
- Sachs, F. Stretch-activated ion channels: What are they? Physiology 2010, 25, 50–56. [Google Scholar] [CrossRef]
- Geffeney, S.L.; Goodman, M.B. How we feel: Ion channel partnerships that detect mechanical inputs and give rise to ouch and pain perception. Neuron 2012, 74, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Richet, C. Contributions à la physiologie des centres nerveux et des muscles de l’écrevisse. Arch. Physiol. Norm. Path. 1879, 6, 263–284, 522–576. (In French) [Google Scholar]
- Richet, C. (Physiologie des Muscles Et des Nerfs: Leçons Professées à la Faculté de Médecine en 1881) par Charles Richet; Paris, G., Ed.; Baillire: Paris, Frence, 1882. (In French) [Google Scholar]
- Huxley, T.H. The Crayfish an Introduction to the Study of Zoology; Series Landmarks of Science; Paul, C.K., Ed.; Kegan Paul, Trench, Trubner & Co., Ltd.: London, UK, 1880. [Google Scholar]
- Wiersma, C.A.G. Vergleichende Untersuchungen über das periphere Nerve-muskel-system von Crustaceen. Zeitschr. vergl. Physiol. 1993, 19, 349–385. [Google Scholar] [CrossRef]
- Van Harreveld, A. A physiological solution for freshwater crustaceans. Proc. Soc. Exp. Biol. Med. 1936, 34, 428–432. [Google Scholar] [CrossRef]
- Prosser, C.L. Effects of salts upon “spontaneous” activity in the nervous system of the crayfish. J. Cell. Comp. Physiol. 1940, 15, 55–65. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Rushton, W.A.H. The electrical constants of a crustacean nerve fibre. Proc. Roy. Soc. 1946, 133, 444–479. [Google Scholar]
- Fatt, P.; Katz, B. Distributed ‘endplate potentials’ of crustacean muscle fibres. J. Exp. Biol. 1953, 30, 433–439. [Google Scholar] [CrossRef]
- Skou, J.C. Enzymatic basis for active transport of Na+ and K+ across cell membrane. Physiol. Rev. 1965, 45, 596–617. [Google Scholar] [CrossRef] [PubMed]
- Skou, J.C. Nobel Lecture. The identification of the sodium pump. Biosci Rep. 1998, 18, 155–169. [Google Scholar] [CrossRef]
- Robbins, J. The excitation and inhibition of crustacean muscle by amino acids. J. Physiol. 1959, 148, 39–50. [Google Scholar] [CrossRef]
- Furshpan, E.J.; Potter, D.D. Transmission at the giant motor synapses of the crayfish. J. Physiol. 1959, 145, 289–325. [Google Scholar] [CrossRef]
- Wiersma, C.A.G.; Hughes, G.M. On the functional anatomy of neuronal units in the abdominal cord of the crayfish, Procambarus clarkii. J. Comp. Neurol. 1961, 116, 209–228. [Google Scholar] [CrossRef]
- Dudel, J.; Kuffler, S.W. Presynaptic inhibition at the crayfish neuromuscular junction. J. Physiol. 1961, 155, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Dudel, J.; Kuffler, S.W. The quantal nature of transmission and spontaneous miniature potentials at the crayfish neuromuscular junction. J. Physiol. 1961, 155, 514–529. [Google Scholar] [CrossRef]
- Alexandrowicz, J.S. Muscle receptor organs in the abdomen of Homarus vulgaris and Palinurus vulgaris. Q. J. Microsc. Sci. 1951, 92, 163–199. [Google Scholar] [CrossRef]
- Kuffler, S.W. Mechanisms of activation and motor control of stretch receptors in lobster and crayfish. J. Neurophysiol. 1954, 17, 558–574. [Google Scholar] [CrossRef]
- Eckert, R.O. Reflex relationships of the abdominal stretch receptors of the crayfish. I. Feedback inhibition of the receptors. J. Cell. Comp. Physiol. 1961, 57, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.O. Reflex relationships of the abdominal stretch receptors of the crayfish. II. Stretch receptor involvement during the swimming reflex. J. Cell. Comp. Physiol. 1961, 57, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Rydqvist, B.; Purali, N. Potential-dependent potassium currents in the rapidly adapting stretch receptor neuron of the crayfish. Acta Physiol. Scand. 1991, 142, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Rydqvist, B.; Swerup, C. Stimulus-response properties of the slowly adapting stretch receptor neuron of the crayfish. Acta Physiol. Scand. 1991, 143, 11–19. [Google Scholar] [CrossRef]
- Bullock, T.; Horridge, G.A. Structure and Function in the Nervous Systems of Invertebrates; Freeman: San Francisco, CA, USA, 1965. [Google Scholar]
- Mill, P.J. (Ed.) Chordotonal organs of crustacean appendages. In Structure and Function of Proprioceptors in the Invertebrates; Chapman and Hall: London, UK, 1976; pp. 243–298. [Google Scholar]
- Alexandrowicz, J.S. Further observations on proprioceptors in Crustacea and a hypothesis about their function. J. Mar. Biol. Assoc. UK 1958, 37, 379–396. [Google Scholar] [CrossRef]
- Alexandrowicz, J.S. Receptor organs in the coxal region of Palinurus vulgaris. J. Mar. Biol. Assoc. UK 1967, 47, 415–432. [Google Scholar] [CrossRef]
- Alexandrowicz, J.S. The comparative anatomy of leg proprioceptors in some decapod Crustacea. J. Mar. Biol. Assoc. UK 1972, 52, 605–634. [Google Scholar] [CrossRef]
- Whitear, M. Chordotonal organs in Crustacea. Nature 1960, 187, 522–523. [Google Scholar] [CrossRef]
- Bush, B.M.H. Proprioceptive reflexes in the legs of Carcinus meanas. J. Exp. Biol. 1962, 39, 89–105. [Google Scholar] [CrossRef]
- Bush, B.M.H. Proprioception by chordotonal organs in the mero-carpopodite and carpo-propodite joints of Carcinus maenas legs. Comp. Biochem. Physiol. 1965, 14, 185–199. [Google Scholar] [CrossRef]
- Bush, B.M.H. Proprioception by the coxo-basal chordotonal organ, CB, in legs of the crab, Carcinus maenas. J. Exp. Biol. 1965, 42, 285–297. [Google Scholar] [CrossRef]
- Cooper, R.L. Proprioceptive neurons of chordotonal organs in the crab, Cancer magister dana (Decapoda, Brachyura). Crustaceana 2008, 81, 447–475. [Google Scholar] [CrossRef]
- Cooper, R.L.; Hartman, H.B. Quantification of responses from proprioceptive neurons in the limbs of the crab, Cancer magister. J. Exp. Zool. 1999, 284, 629–636. [Google Scholar] [CrossRef]
- Hartman, H.B.; Boettiger, E.G. The functional organization of the propus-dactylus organ in Cancer irroratus Say. Comp. Biochem. Physiol. 1967, 22, 651–663. [Google Scholar] [CrossRef]
- Atkins, D.E.; Bosh, K.L.; Breakfield, G.W.; Daniels, S.E.; Devore, M.J.; Fite, H.E.; Guo, L.Z.; Henry, D.K.J.; Kaffenberger, A.K.; Manning, K.S.; et al. The Effect of Calcium Ions on Mechanosensation and Neuronal Activity in Proprioceptive Neurons. NeuroSci 2021, 2, 26. [Google Scholar] [CrossRef]
- Dayaram, V.; Malloy, C.; Martha, S.; Alvarez, B.; Chukwudolue, I.; Dabbain, N.; Dmahmood, D.; Goleva, S.; Hickey, T.; Ho, A.; et al. The effect of CO2, intracellular pH and extracellular pH on mechanosensory proprioceptor responses in crayfish and crab. Am. J. Undergrad. Res. 2017, 14, 85–99. [Google Scholar] [CrossRef]
- McCubbin, S.; Jeoung, A.; Waterbury, C.; Cooper, R.L. Pharmacological profiling of stretch activated channels in proprioceptive neuron. Comp. Biochem. Physiol. C 2020, 233, 108765. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.E.; Adams, R.; Nadolski, J.; Amrit, E.; Barrett, M.; Bohnett, C.; Campbell, K.; Deweese, K.; Dhar, S.; Gillis, B.; et al. The effects of tricaine mesylate on arthropods: Crayfish, crab and Drosophila. Invertebr. Neurosci. 2020, 20, 10. [Google Scholar] [CrossRef]
- Pankau, C.; Nadolski, J.; Tanner, H.; Cryer, C.; Di Girolamo, J.; Haddad, C.; Lanning, M.; Miller, M.; Neely, D.; Wilson, R.; et al. Examining the effect of manganese on physiological processes: Invertebrate models. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 251, 109209. [Google Scholar] [CrossRef] [PubMed]
- Tanner, H.N.; Atkins, D.E.; Bosh, K.L.; Breakfield, G.W.; Daniels, S.E.; Devore, M.J.; Fite, H.E.; Guo, L.Z.; Henry, D.K.J.; Kaffenberger, A.K. Effect of TEA and 4-AP on primary sensory neurons in a crustacean model. J. Pharmacol. Toxicol. 2022, 17, 14–27. [Google Scholar] [CrossRef]
- Ison, B.J.; Abul-Khoudoud, M.O.; Ahmed, S.; Alhamdani, A.W.; Ashley, C.; Bidros, P.C.; Bledsoe, C.O.; Bolton, K.E.; Capili, J.G.; Henning, J.N.; et al. The effect of doxapram on proprioceptive neurons: Invertebrate model. NeuroSci 2022, 3, 41. [Google Scholar] [CrossRef]
- O’Neil, A.S.; Krall, R.M.; Vascassenno, R.; Cooper, R.L. Exploring mechanisms in a medical treatment for a disease: A teaching/learning module. Advances in Biology Laboratory Education. Publ. Assoc. Biol. Lab. Educ. ABLE 2023, 43, 35. [Google Scholar]
- Majeed, Z.R.; Titlow, J.; Hartman, H.B.; Cooper, R.L. Proprioception and tension receptors in crab limbs: Student laboratory exercises. J. Vis. Exp. 2013, 80, e51050. [Google Scholar]
- Whitear, M. The fine structure of crustacean proprioceptors. I. The chordotonal organs in the legs of the shore crab, Carcinus meanas. Phil. Trans. Roy. Soc. Lond. B 1962, 245, 291–325. [Google Scholar]
- Whitear, M. The fine structure of crustacean proprioceptors. II. The thoracico-coxal organs in Carcinus, Pagurus and Astacus. Phil. Trans. Roy. Soc. Lond. B 1965, 248, 437–462. [Google Scholar]
- De Col, R.; Messlinger, K.; Carr, R.W. Conduction velocity is regulated by sodium channel inactivation in unmyelinated axons innervating the rat cranial meninges. J. Physiol. 2008, 586, 1089–1103. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E. The effect of metabolic and ion transport inhibitors on the impulse activity and oxygen uptake of an isolated crustacean neurone. Acta Physiol. Scand. 1966, 66, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Rybakowski, J. Lithium treatment—The state of the art for 2020. Psychiatr. Pol. 2020, 54, 1047–1066, (In English, In Polish). [Google Scholar] [CrossRef]
- Malhi, G.S.; Tanious, M.; Das, P.; Coulston, C.M.; Berk, M. Potential mechanisms of action of lithium in bipolar disorder. Current understanding. Current understanding. CNS Drugs. 2013, 27, 135–153. [Google Scholar] [CrossRef]
- Richardson, J.; Kotevski, A.; Poole, K. From stretch to deflection: The importance of context in the activation of mammalian, mechanically activated ion channels. FEBS J. 2022, 289, 4447–4469. [Google Scholar] [CrossRef] [PubMed]
- Kamuene, J.M.; Xu, Y.; Plant, L.D. The pharmacology of two-pore domain potassium channels. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2021; Volume 267, pp. 417–443. [Google Scholar]
- Sepúlveda, F.V.; Pablo Cid, L.; Teulon, J. Niemeyer MI. Molecular aspects of structure, gating, and physiology of pH-sensitive background K2P and Kir+-transport channels. Physiol. Rev. 2015, 95, 179–217. [Google Scholar] [CrossRef]
- Mita, K.; Sumikama, T.; Iwamoto, M.; Matsuki, Y.; Shigemi, K.; Oiki, S. Conductance selectivity of Na+ across the K+ channel via Na+ trapped in a tortuous trajectory. Proc. Natl. Acad. Sci. USA 2021, 118, e2017168118. [Google Scholar] [CrossRef] [PubMed]










| Preparation | Amplitude of CAP | Conduction Velocity | Trend to Recover in Saline Rinse |
|---|---|---|---|
| 1 | ↓ | ↓ | + |
| 2 | ↓ | ↓ | + |
| 3 | ↓ | ↓ | + |
| 4 | ↓ | ↓ | + |
| 5 | ↓ | ↓ | + |
| 6 | ↓ | ↓ | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brock, K.E.; Elliott, E.R.; Taul, A.C.; Asadipooya, A.; Bocook, D.; Burnette, T.; Chauhan, I.V.; Chhadh, B.; Crane, R.; Glover, A.; et al. The Effects of Lithium on Proprioceptive Sensory Function and Nerve Conduction. NeuroSci 2023, 4, 280-295. https://doi.org/10.3390/neurosci4040023
Brock KE, Elliott ER, Taul AC, Asadipooya A, Bocook D, Burnette T, Chauhan IV, Chhadh B, Crane R, Glover A, et al. The Effects of Lithium on Proprioceptive Sensory Function and Nerve Conduction. NeuroSci. 2023; 4(4):280-295. https://doi.org/10.3390/neurosci4040023
Chicago/Turabian StyleBrock, Kaitlyn E., Elizabeth R. Elliott, Alaina C. Taul, Artin Asadipooya, Devin Bocook, Tessa Burnette, Isha V. Chauhan, Bilal Chhadh, Ryan Crane, Ashley Glover, and et al. 2023. "The Effects of Lithium on Proprioceptive Sensory Function and Nerve Conduction" NeuroSci 4, no. 4: 280-295. https://doi.org/10.3390/neurosci4040023