Structural and Dynamic Behaviour of Heterocycles Derived from Ethylenediamines with Formaldehyde: 1,3,5-Triazinanes and Bis(imidazolidinyl)methanes
Abstract
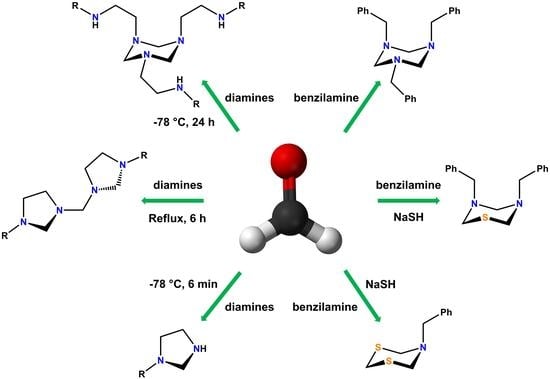
:1. Introduction
2. Materials and Methods
2.1. General Experimental Details
2.2. Synthesis of 1,3,5-Triazinanes (1a-1d)
2.2.1. Synthesis of 2,2′,2″-(1,3,5-Triazinane-1,3,5-triyl)tris(N-methylethylenamine) (1a)
2.2.2. Synthesis of 2,2′,2″-(1,3,5-Triazinane-1,3,5-triyl)tris(N-ethylethylenamine) (1b)
2.2.3. Synthesis of 2,2′,2″-(1,3,5-Triazinane-1,3,5-triyl)tris(N-benzylethylenmine) (1c)
2.2.4. Synthesis of 2,2′,2″-(1,3,5-Triazinane-1,3,5-triyl)tris(N,N-dimethylethylenamine) (1d)
2.3. Synthesis of Bis(3-alkyl-imidazolidin-1-yl)methane (2a-2d)
2.3.1. Obtaining Bis(3-methylimidazolidin-1-yl)methane (2a)
2.3.2. Obtaining Bis(3-ethylimidazolidin-1-yl)methane (2b)
2.3.3. Obtaining Bis(3-benzylimidazolidin-1-yl)methane (2c)
2.3.4. Obtaining Bis(3-phenylimidazolidin-1-yl)methane (2d)
2.4. Synthesis of 1,3,5-Tribenzyl-1,3,5-triazinane (3)
2.5. Synthesis of 3,5-Dibenzyl-1,3,5-thiadiazinane (4)
2.6. Synthesis of 5-Benzyl-1,3,5-dithiazinane (5)
3. Results and Discussion
3.1. Preparation of 1,3,5-Triazinanes (1a-1d)
3.2. Preparation of Bis(imidazolidinyl)methanes (2a-2d)
3.3. NMR Reaction Evaluation
3.4. Spectroscopic Characterization of 1,3,5-Triazinanes (1a-1d)
3.5. Spectroscopic Characterization of Bis(imidazolidinyl)methanes (2a-2d)
3.6. Spectrometric Characterization of Bis(imidazolidinyl)methanes (2a-2d)
3.7. Versatility of Formaldehyde in the Synthesis of Six-Member Heterocycles
3.8. Crystallographic Characterization of N-Benzyl-triazinane (3) and N-Benzyl-thiadiazinane (4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- da Silva Junior, A.A.; da Rocha Pitta, M.G.; de Oliveira Chagas, M.B.; Barreto de Melo Rêgo, M.J.; da Rosa, M.M.; da Rocha Pitta, M.G. Imidazolidine derivatives in cancer research: What is known? Anticancer. Agents Med. Chem. 2022, 22, 1272–1277. [Google Scholar] [CrossRef]
- Swain, S.P.; Mohanty, S. Imidazolidinones and imidazolidine-2,4-diones as antiviral agents. ChemMedChem 2019, 14, 291–302. [Google Scholar] [CrossRef]
- Perillo, I.; Repetto, E.; Caterina, M.C.; Massa, R.; Gutkind, G.; Salerno, A. Synthesis, spectroscopic and biological properties of bis(3-arylimidazolidinyl-1)methanes. A novel family of antimicrobial agents. Eur. J. Med. Chem. 2005, 40, 811–815. [Google Scholar] [CrossRef]
- Caterina, M.C.; Perillo, I.A.; Boiani, L.; Pezaroglo, H.; Cerecetto, H.; Gonzalez, M.; Salerno, A. Imidazolidines as new anti-Trypanosoma cruzi agents: Biological evaluation and structure-activity relationships. Bioorganic Med. Chem. 2008, 16, 2226–2234. [Google Scholar] [CrossRef]
- Farzaliev, V.M.; Babaeva, G.B.; Abbasova, M.T.; Nabiev, O.G.; Soltanova, Z.K.; Kerimova, Y.M. Derivatives of N-alkyl(aryl)-1,2(1,3)-diazacycloalkanes. Antimicrobial properties. Chem. Technol. Fuels Oil 2009, 45, 98–102. [Google Scholar] [CrossRef]
- Guerra, A.; Gonzalez-Naranjo, P.; Campillo, N.E.; Cerecetto, H.; Gonzalez, M.; Paez, J.A. Artificial neural networks based on CODES descriptors in pharmacology: Identification of novel trypanocidal drugs against Chagas disease. Curr. Comput.-Aided Drug Des. 2013, 9, 130–140. [Google Scholar] [CrossRef]
- Caterina, M.C.; Perillo, I.A.; Villalonga, X.; Amiano, N.; Payés, C.; Sanchez, M.L.; Salerno, A. New green synthesis and antineoplastic activity of bis(3-arylimidazolidinyl-1)methane. Open J. Med. Chem. 2013, 3, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Kamps, I.; Mix, A.; Berger, R.J.F.; Neumann, B.; Stammler, H.-G.; Mitzel, N.W. Two diamino-substituted lithiocarbanions in one molecule. Chem. Commun. 2009, 5558–5560. [Google Scholar] [CrossRef] [PubMed]
- Salas-Coronado, R.; Galvez-Ruiz, J.C.; Jaen-Gaspar, J.G.; Noth, H.; Flores-Parra, A. 3-(1,3-Heterazolidin-3-yl-methyl)-1,3-oxazolidines and their reduction with borane-THF. J. Mol. Struct. THEOCHEM 2003, 640, 95–108. [Google Scholar] [CrossRef]
- Reis, M.I.P.; Romeiro, G.A.; Damasceno, R.; da Silva, F.d.C.; Ferreira, V.F. Synthesis and applications of 1,3,5-triazinanes. Rev. Virt. Quim. 2013, 5, 283–299. [Google Scholar] [CrossRef]
- Yan, Z.; Xue, W.-L.; Zeng, Z.-X.; Gu, M.-R. Kinetics of cyanuric chloride hydrolysis in aqueous solution. Ind. Eng. Chem. Res. 2008, 47, 5318–5322. [Google Scholar] [CrossRef]
- Puthiaraj, P.; Kim, S.-S.; Ahn, W.-S. Covalent triazine polymers using a cyanuric chloride precursor via Friedel-Crafts reaction for CO2 adsorption/separation. Chem. Eng. J. 2016, 283, 184–192. [Google Scholar] [CrossRef]
- Basu, N.; Maity, S.K.; Chaudhury, A.; Ghosh, R. Trichloroisocyanuric acid (TCCA): An efficient green reagent for activation of thioglycosides toward hydrolysis. Carbohydr. Res. 2013, 369, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Combe, S.H.; Hosseini, A.; Parra, A.; Schreiner, P.R. Mild aliphatic and benzylic hydrocarbon C-H bond chlorination using trichloroisocyanuric acid. J. Org. Chem. 2017, 82, 2407–2413. [Google Scholar] [CrossRef] [PubMed]
- Kukharev, B.F.; Stankevich, V.K.; Klimenko, G.R.; Lobanova, N.A.; Kovalyuk, E.N.; Negoda, A.Y.; Stankevich, V.V.; Bragin, E.V. Anticorrosion properties of products of N-(2-vinyloxyethyl)-1,2-ethylenediamine condensation with carbonyl compounds. Russ. J. Appl. Chem. 2010, 83, 1666–1667. [Google Scholar] [CrossRef]
- Onyeachu, I.B.; Chauhan, D.S.; Quraishi, M.A.; Obot, I.B. Influence of hydrodynamic condition on 1,3,5-tris(4-methoxyphenyl)-1,3,5-triazinane as a novel corrosion inhibitor formulation for oil and gas industry. Corros. Eng. Sci. Technol. 2021, 56, 154–161. [Google Scholar] [CrossRef]
- Divya, R.; Nair, L.P.; Bijini, B.R.; Nair, C.M.K.; Babu, K.R. Growth and characterization of barium complex of 1,3,5-triazinane-2,4,6-trione in gel: A corrosion inhibiting material. Appl. Phys. A Mater. Sci. Process. 2018, 124, 399. [Google Scholar] [CrossRef]
- He, Z.; Meng, T.; Wang, Y.; Guo, Z.; Liu, F.; Liu, Z. Effect of 2,4,6-triamino-3,5-dinitropyridine-1-oxide on the properties of 1,3,5-trinitro-1,3,5-triazinane-based PBX explosives. Propellants Explos. Pyrotech. 2021, 46, 530–536. [Google Scholar] [CrossRef]
- Morrison, K.A.; Denis, E.H.; Nims, M.K.; Broderick, A.M.; Fausey, R.C.; Rose, H.J.; Gongwer, P.E.; Ewing, R.G. Vapor pressures of RDX and HMX explosives measured at and near room temperature: 1,3,5-trinitro-1,3,5-triazinane and 1,3,5,7-tetranitro-1,3,5,7-tetrazocane. J. Phys. Chem. A 2021, 125, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wu, Y.; Wang, K.; Sun, Y.; Zhu, H.; Yu, J.; Xu, C. Process improvements for the preparation of insecticide clothianidin. Asian J. Chem. 2014, 26, 2815–2819. [Google Scholar] [CrossRef]
- Liang, D.; Xiao, W.-J.; Chen, J.-R. Recent advances of 1,3,5-triazinanes in aminomethylation and cycloaddition reactions. Synthesis 2020, 52, 2469–2482. [Google Scholar] [CrossRef]
- Gong, J.; Li, S.-W.; Qurban, S.; Kang, Q. Enantioselective Mannich reaction employing 1,3,5-triaryl-1,3,5-triazinanes catalyzed by chiral-at-metal rhodium complexes. Eur. J. Org. Chem. 2017, 2017, 3584–3593. [Google Scholar] [CrossRef]
- Kickelbick, G.; Gallauner, T. A Mixed copper(I)/copper(II) complex coordinated by a multidentate amidato ligand. Acta Crystallogr. E 2002, 58, m102–m104. [Google Scholar] [CrossRef]
- Colorado-Peralta, R.; Lopez-Rocha, C.A.; Sanchez-Ruiz, S.A.; Contreras, R.; Flores-Parra, A. New dithiazinanes and bis-dithiazinanes-bearing pendant ethylamines: Structure and reactivity. Heteroatom Chem. 2011, 22, 59–71. [Google Scholar] [CrossRef]
- Kickelbick, G.; Rutzinger, D.; Gallauner, T. Synthesis of hexadentate hexahydro-1,3,5-triazine-based ligands and their copper(I) complexes. Monatsh. Chem. 2002, 133, 1157–1164. [Google Scholar] [CrossRef]
- Witanowski, M.; Stefaniak, L.; Januszewski, H. Nitrogen Chemical Shifts in Organic Compounds; Nitrogen, N.M.R., Witanowski, M., Webb, G.A., Eds.; Springer: Boston, MA, USA, 1973; pp. 163–260. [Google Scholar] [CrossRef]
- Webb, G.A.; Nitrogen, N.M.R. Encyclopedia of Spectroscopy and Spectrometry; Lindon, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 1504–1514. [Google Scholar] [CrossRef]
- Gao, P.; Wang, X.; Yu, H. Towards an accurate prediction of nitrogen chemical shifts by density functional theory and gauge-including atomic orbital. Adv. Theory Simul. 2019, 2, 1800148. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Zhou, S.-J.; Xu, G.-Y.; Wang, L.; Yang, Q.-Q.; Xuan, J. [4 + 2]-Cycloaddition of para-quinone methides with hexahydro-1,3,5-triazines: Access to 1,3-benzoxazine derivatives. Adv. Synth. Catal. 2020, 362, 523–527. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, B.; Li, H.; He, Y.; Xu, W.; Duan, X.; Sun, H.; Wang, T.; Zhai, H. Synthesis of hydrobenzoimidazoles from para-quinamines and 1,3,5-triazinanes via a Formal [3+2] annulation reaction. Adv. Synth. Catal. 2021, 363, 565–569. [Google Scholar] [CrossRef]
- Liang, D.; Tan, L.-P.; Xiao, W.-J.; Chen, J.-R. Inverse-electron-demand [4 + 2] cycloaddition of photogenerated aza-ortho-quinone methides with 1,3,5-triazinanes: Access to perfluoroalkylated tetrahydroquinazolines. Chem. Commun. 2020, 56, 3777–3780. [Google Scholar] [CrossRef]
- Cheng, B.; Zhang, X.; Zhai, S.; He, Y.; Tao, Q.; Li, H.; Wei, J.; Sun, H.; Wang, T.; Zhai, H. Synthesis of 1,2,3,4-tetrahydrobenzofuro[3,2-d]pyrimidines via [4 + 2] annulation reaction of 1,3,5-triazinanes and aurone-derived α,β-unsaturated imines. Adv. Synth. Catal. 2020, 362, 3836–3840. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, B.; Hu, L.; Sun, H.; Wang, Y.; Zhai, H.; Cheng, B. Synthesis of chromeno[2,3-d]pyrimidin-5-one derivatives from 1,3,5-triazinanes via two different reaction pathways. J. Org. Chem. 2022, 87, 1348–1356. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Zheng, H.; Huang, J.; Su, Z.; Zhao, L.; Cao, H. Construction of diverse N-heterocycles by formal (3 + 3) cycloaddition of naphthol/thionaphthol/naphthylamine and 1,3,5-triazinanes. J. Org. Chem. 2023, 88, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhong, Q.; Tang, S.; Wang, L.; Li, P.; Li, H. Electrochemical formal [3 + 2] cycloaddition of azobenzenes with hexahydro-1,3,5-triazines. Org. Chem. Front. 2022, 9, 3769–3774. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, A.; Liu, X.; Yu, Y.; Zhu, B.; Cao, H. Lewis acid-catalyzed synthesis of polysubstituted furans from conjugated ene-yne-ketones and 1,3,5-triazinanes. J. Org. Chem. 2022, 87, 7056–7063. [Google Scholar] [CrossRef]
- Kireeva, D.R.; Sadretdinov, S.S.; Musina, A.I.; Ishmetova, D.V.; Vakhitov, V.A.; Murinov, Y.I.; Dokichev, V.A. Synthesis and cytotoxic activity of 1,3,5-triazinane derivatives based on primary amines and amino acids esters. Russ. J. Gen. Chem. 2022, 92, 24–28. [Google Scholar] [CrossRef]
- Fang, Z.; Jin, Q.; Wang, X.; Ning, Y. Metal-free [2 + 1 + 3] Cycloaddition of trifluoroacetaldehyde N-sulfonylhydrazones with hexahydro-1,3,5-triazines leading to trifluoromethylated 2,3,4,5-tetrahydro-1,2,4-triazines. J. Org. Chem. 2022, 87, 2966–2974. [Google Scholar] [CrossRef]
- Ruan, P.; Tang, Q.; Yang, Z.; Liu, X.; Feng, X. Enantioselective formal [2 + 2 + 2] cycloaddition of 1,3,5-triazinanes to construct tetrahydropyrimidin-4-one derivatives. Chem. Commun. 2022, 58, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Gao, L.; Chen, W.; Shi, Y.; Cao, Z.; Zheng, Y.; Liu, J. Formal [2+2+2] cycloaddition reaction of 1,3,5-triazinanes with diethyl acetylenedicarboxylate: Approach to tetrahydropyrimidines. Eur. J. Org. Chem. 2021, 2021, 5941–5945. [Google Scholar] [CrossRef]
- Wang, C.; Fang, L.; Wang, Z. Base-induced inverse-electron-demand aza-Diels-Alder reaction of azoalkenes and 1,3,5-triazinanes: Facile approaches to tetrahydro-1,2,4-triazines. Tetrahedron Lett. 2021, 79, 153303. [Google Scholar] [CrossRef]
- Liu, L.; Shi, Z.; Zhang, X.; Zhan, F.; Lin, J.-S.; Jiang, Y. Synthesis of α-amino tertiary alkylperoxides by Lewis acid-catalyzed peroxidation of 1,3,5-triazines. Chem. Asian J. 2021, 16, 3487–3491. [Google Scholar] [CrossRef]
- Duan, S.; Meng, H.; Jablasone, S.T., Jr.; Luo, H.; Xu, Z.-F.; Li, C.-Y. Rhodium(II)-catalyzed [4 + 2] annulation of ester-tethered 1-sulfonyl-1,2,3-triazoles and hexahydro-1,3,5-triazines. Asian J. Org. Chem. 2021, 10, 1076–1080. [Google Scholar] [CrossRef]
- Galvez-Ruiz, J.C.; Jaen-Gaspar, J.C.; Castellanos-Arzola, I.G.; Contreras, R.; Flores-Parra, A. 2-(1,3,5-Dithiazinan-5-yl)ethanol heterocycles, structure and reactivity. Heterocycles 2004, 63, 2269–2285. [Google Scholar] [CrossRef]
- Winfield, L.; Zhang, C.; Reid, C.A.; Stevens, E.D.; Trudell, M.L.; Izenwasser, S.; Wade, D. Synthesis, lipophilicity and structure of 2,5-disubstituted 1,3,5-dithiazine derivatives. J. Heterocycl. Chem. 2003, 40, 827–832. [Google Scholar] [CrossRef]
- Khabibullina, G.R.; Yapparova, D.K.; Ibragimov, A.G.; Akhmetova, V.R. Sodium sulfide in the synthesis of N-Alkyl-1,3,5-dithiazinanes and 1,3,5-thiadiazinanes. Russ. J. Gen. Chem. 2021, 91, 1453–1458. [Google Scholar] [CrossRef]
- Peerzada, N.; Neely, I. Benzotriazole mediated synthesis of some 5-alkyldihydro-4H-1,3,5-dithiazines. Synth. Commun. 2000, 30, 779–788. [Google Scholar] [CrossRef]
- Xotlanihua-Flores, A.; Montes-Tolentino, P.; Sánchez-Ruiz, S.A.; Suárez-Moreno, G.V.; Gálvez-Ruiz, J.C.; Contreras, R.; Flores-Parra, A. New N-[2-chloropropyl]-heterocyclohexanes. NMR long range shielding effects of chlorine substituent. Use of BH3 as freezing conformational agent. J. Mol. Struct. 2016, 1106, 322–330. [Google Scholar] [CrossRef]
- Xotlanihua-Flores, A.; Villaseñor-Granados, T.O.; Montes-Tolentino, P.; Flores-Parra, A. New 1,3,5-heterocyclohexanes bearing pendant phosphorus groups. Structure and N→P pnicogen interactions. J. Mol. Struct. 2022, 1252, 131916. [Google Scholar] [CrossRef]
- Xotlanihua-Flores, A.; Villaseñor-Granados, T.O.; Colorado-Peralta, R.; Sánchez-Ruiz, S.A.; Montes-Tolentino, P.; Flores-Parra, A. Tin complexes derived from nitrogen-based 1,3,5-heterocyclohexanes bearing 2-hydroxypropan-1-yl, 2-diphenylphosphitepropan-1-yl and 2-diphenylphosphinepropan-1-yl as pendant N-substituents. J. Mol. Struct. 2022, 1254, 132368. [Google Scholar] [CrossRef]
- Suarez-Moreno, G.V.; Xotlanihua-Flores, A.; Vela, A.; Contreras, R.; Flores-Parra, A. Theoretical approach to the conformational analyses of dithiazinane, thiadiazinane and triazinane, their N-borane adducts and N-H cations. J. Mol. Struct. 2016, 1113, 112–126. [Google Scholar] [CrossRef]











| Reagents (Eq.) | Reaction Conditions | Products (%) | |||||
|---|---|---|---|---|---|---|---|
| A | B | Solvent | Temperature | Time | C | D | E |
| 1 | 1 | THF-d8 | −78 °C | 6 min (0.1 h) | 42 | 21 | 37 |
| 1 | 1 | THF-d8 | 25 °C | 360 min (6 h) | 21 | 43 | 36 |
| 1 | 1 | THF-d8 | 25 °C | 1440 min (24 h) | 0 | 40 | 60 |
| 1 | 1 | THF-d8 | 65 °C | 360 min (6 h) | 0 | 0 | 100 |
| 1 | 1 | DMSO-d6 | 5 °C | 6 min (0.1 h) | 25 | 39 | 36 |
| 1 | 1 | DMSO-d6 | 25 °C | 6 min (0.1 h) | 21 | 43 | 36 |
| 1 | 1 | DMSO-d6 | 120 °C | 60 min (1 h) | 0 | 26 | 74 |
| 2 | 1 | DMSO-d6 | 5 °C | 6 min (0.1 h) | 71 | 17 | 12 |
| 2 | 1 | DMSO-d6 | 120 °C | 150 min (2.5 h) | 0 | 50 * | 0 |
| 1 | 2 | DMSO-d6 | 5 °C | 6 min (0.1 h) | 1 | 1 | 98 |
| 1 | 2 | DMSO-d6 | 120 °C | 90 min (1.5 h) | 0.5 | 0.5 | 99 |
| Prod. | C2, C4, C6 | C7 | C8 | R Group | H2, H4, H6 | H7 | H8 | R Group |
|---|---|---|---|---|---|---|---|---|
| 1a | 72.3 | 54.2 | 45.7 | 39.5, (Me) | 3.29 (s) | 2.50 (t) J 7.0 | 2.96 (t) J 7.0 | 2.25 (s), (Me) |
| 1b | 70.3 | 51.9 | 45.2 | 47.5, 14.1, (Et) | 3.24 (s) | 2.42 (t) J 7.0 | 2.86 (t) J 7.0 | 2.31 (c), 0.92 (t), (Et) |
| 1c | 70.7 | 52.3 | 45.4 | 58.0, 139.0, 128.7, 128.5, 127.2, (CH2Ph) | 3.42 (s) | 2.62 (t) J 7.1 | 3.02 (t) J 7.1 | 3.59 (s), 7.4–7.2 (m), (CH2Ph) |
| 1d | 74.7 | 50.4 | 57.5 | 45.6 (Me) | 3.12 (s) | 2.29 (t) J 6.8 | 2.12 (t) J 6.8 | 1.96 (s), (Me) |
| N-Alkyl- Ethylenediamine | N1 | N4 | 1,3,5-Triazinane | N1, N3, N5 | N9 |
|---|---|---|---|---|---|
| a | −363.5 | −360.8 | 1a | −340.0 | −327.1 |
| b | −362.9 | −341.2 | 1b | −341.2 | −326.1 |
| c | −362.0 | −343.3 | 1c | −340.9 | −318.8 |
| d | −363.5 | −360.3 | 1d | −334.1 | −355.6 |
| Prod. | C2 | C6 | C4 | C5 | R Group | H2 | H6 | H4 | H5 | R Group |
|---|---|---|---|---|---|---|---|---|---|---|
| 2a | 76.6 | 77.0 | 54.1 | 51.0 | 40.6, (Me) | 3.26 (s) | 3.23 (s) | 2.55 (t) J 6.7 | 2.75 (t) J 6.7 | 2.19 (s), (Me) |
| 2b | 74.6 | 76.4 | 51.7 | 50.3 | 48.5, 13.7, (Et) | 3.09 (s) | 3.01 (s) | 2.36 (t) J 6.6 | 2.53 (t) J 6.6 | 2.14 (c), 0.73 (t), (Et) |
| 2c | 75.2 | 76.7 | 52.3 | 50.7 | 59.1, 139.1, 128.7, 128.4, 127.1, (CH2Ph) | 3.42 (s) | 3.33 (s) | 2.70 (t) J 7.2 | 2.86 (t) J 7.2 | 3.60 (s), 7.4–7.2 (m), (CH2Ph) |
| 2d | 69.1 | 74.5 | 45.8 | 51.0 | 146.7, 129.4, 116.5, 111.8, (Ph) | 4.17 (s) | 3.47 (s) | 3.42 (t) J 6.6 | 3.13 (t) J 6.6 | 7.3–6.5 (m), (Ph) |
| N-Alkyl- Ethylenediamine | N1 | N4 | Bis(imidizalodinyl) Methanes | N1 | N3 |
|---|---|---|---|---|---|
| a | −363.5 | −360.8 | 2a | −326.9 | −340.7 |
| b | −362.9 | −341.2 | 2b | −327.2 | −328.8 |
| c | −362.0 | −343.3 | 2c | −327.6 | −327.9 |
| d | −361.8 | −316.7 | 2d | −325.7 | −309.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colorado-Peralta, R.; Sánchez-Ruiz, S.A.; Flores-Parra, A. Structural and Dynamic Behaviour of Heterocycles Derived from Ethylenediamines with Formaldehyde: 1,3,5-Triazinanes and Bis(imidazolidinyl)methanes. Organics 2023, 4, 297-312. https://doi.org/10.3390/org4020024
Colorado-Peralta R, Sánchez-Ruiz SA, Flores-Parra A. Structural and Dynamic Behaviour of Heterocycles Derived from Ethylenediamines with Formaldehyde: 1,3,5-Triazinanes and Bis(imidazolidinyl)methanes. Organics. 2023; 4(2):297-312. https://doi.org/10.3390/org4020024
Chicago/Turabian StyleColorado-Peralta, Raúl, Sonia Araceli Sánchez-Ruiz, and Angelina Flores-Parra. 2023. "Structural and Dynamic Behaviour of Heterocycles Derived from Ethylenediamines with Formaldehyde: 1,3,5-Triazinanes and Bis(imidazolidinyl)methanes" Organics 4, no. 2: 297-312. https://doi.org/10.3390/org4020024