Unraveling the Synthesis of SbCl(C3N6H4): A Metal-Melaminate Obtained through Deprotonation of Melamine with Antimony(III)Chloride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Synthesis
- Synthesis of SbCl4(C9N18H19) (1):
- Synthesis of (SbCl4(C6N12H13))2 (2):
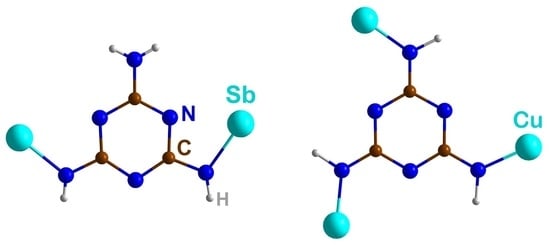
- Synthesis of SbCl(C3N6H4) (3):
2.1.2. X-ray Powder Diffraction
2.1.3. Single-Crystal X-ray Diffraction
2.1.4. Thermoanalytic Studies
2.1.5. Infrared Spectra
3. Results and Discussion
3.1. Thermoanalytic Studies
3.2. Crystal Structures
3.3. X-ray Powder Diffraction and Infrared Spectroscopy
3.4. Infrared Spectroscopy (IR)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liebig, J. Über einige Stickstoff-Verbindungen. Ann. Pharm. Fr. 1834, 10, 1–47. [Google Scholar] [CrossRef]
- Gmelin, L. Über einige Verbindungen des Melon’s. Ann. Pharm. Fr. 1835, 15, 252–258. [Google Scholar] [CrossRef]
- Liebig, J. Über die Constitution der Mellonverbindungen. Justus Liebigs Ann. Chem. 1855, 95, 257–282. [Google Scholar] [CrossRef]
- Finkel’shtein, A.; Boitsov, E. The molecular structure of 1,3,5-triazine and its derivatives. Russ. Chem. Rev. 1962, 31, 712. [Google Scholar] [CrossRef]
- Crews, G.M.; Ripperger, W.; Kersebohm, D.B.; Güthner, T.; Mertschenk, B. Melamine and guanamines. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Weinheim, Germany, 2001. [Google Scholar] [CrossRef]
- Keßler, F.K. Structure and Reactivity of S-Triazine-Based Compounds in C/N/H Chemistry. Ph.D. Thesis, Ludwig Maximilian University of Munich, Munich, Germany, 2019. [Google Scholar]
- Klason, P. Über Melamverbindungen. J. Prakt. Chem. 1886, 33, 285–289. [Google Scholar] [CrossRef]
- Finkel’shtein, A.I.; Spiridonova, N.Y.V. Chemical properties and molecular structure of derivatives of sym-heptazine [1,3,4,6,7,9,9b-heptaazaphenalene, tri-1,3,5-triazine]. Russ. Chem. Rev. 1964, 33, 400. [Google Scholar] [CrossRef]
- Jürgens, B.; Irran, E.; Senker, J.; Kroll, P.; Müller, H.; Schnick, W. Melem (2,5,8-Triamino-tri-s-triazine), an Important Intermediate during Condensation of Melamine Rings to Graphitic Carbon Nitride: Synthesis, Structure Determination by X-ray Powder Diffractometry, Solid-State NMR, and Theoretical Studies. J. Am. Chem. Soc. 2003, 125, 10288–10300. [Google Scholar] [CrossRef]
- Kroke, E.; Schwarz, M. Novel group 14 nitrides. Coord. Chem. Rev. 2004, 248, 493–532. [Google Scholar] [CrossRef]
- May, H. Pyrolysis of melamine. J. Appl. Chem. 1959, 9, 340–344. [Google Scholar] [CrossRef]
- Sattler, A.; Pagano, S.; Zeuner, M.; Zurawski, A.; Gunzelmann, D.; Senker, J.; Müller-Buschbaum, K.; Schnick, W. Melamine–melem adduct phases: Investigating the thermal condensation of melamine. Chem.-Eur. J. 2009, 15, 13161–13170. [Google Scholar] [CrossRef]
- Lotsch, B.V.; Schnick, W. New light on an old story: Formation of melam during thermal condensation of melamine. Chem.-Eur. J. 2007, 13, 4956–4968. [Google Scholar] [CrossRef] [PubMed]
- Weil, E.D. Fire-Protective and Flame-Retardant Coatings—A State-of-the-Art Review. J. Fire Sci. 2011, 29, 259–296. [Google Scholar] [CrossRef]
- Schartel, B.; Weiß, A.; Mohr, F.; Kleemeier, M.; Hartwig, A.; Braun, U. Flame retarded epoxy resins by adding layered silicate in combination with the conventional protection-layer-building flame retardants melamine borate and ammonium polyphosphate. J. Appl. Polym. Sci. 2010, 118, 1134–1143. [Google Scholar] [CrossRef]
- Yang, H.; Song, L.; Tai, Q.; Wang, X.; Yu, B.; Yuan, Y.; Hu, Y.; Yuen, R.K.K. Comparative study on the flame retarded efficiency of melamine phosphate, melamine phosphite and melamine hypophosphite on poly(butylene succinate) composites. Polym. Degrad. Stab. 2014, 105, 248–256. [Google Scholar] [CrossRef]
- Weil, E.D.; Levchik, S.V. Flame Retardants in Commercial Use or Development for Textiles. J. Fire Sci. 2008, 26, 243–281. [Google Scholar] [CrossRef]
- Yin, N.; Wang, K.; Xia, Y.a.; Li, Z. Novel melamine modified metal-organic frameworks for remarkably high removal of heavy metal Pb(II). Desalination 2018, 430, 120–127. [Google Scholar] [CrossRef]
- Cao, Y.; Huang, J.; Li, Y.; Qiu, S.; Liu, J.; Khasanov, A.; Khan, M.A.; Young, D.P.; Peng, F.; Cao, D.; et al. One-pot melamine derived nitrogen doped magnetic carbon nanoadsorbents with enhanced chromium removal. Carbon 2016, 109, 640–649. [Google Scholar] [CrossRef]
- Kallenbach, P.; Bayat, E.; Ströbele, M.; Romao, C.P.; Meyer, H.-J. Tricopper Melaminate, a Metal-Organic Framework Containing Dehydrogenated Melamine and Cu-Cu Bonding. Inorg. Chem. 2021, 60, 16303–16307. [Google Scholar] [CrossRef]
- Pareek, K.; Rohan, R.; Cheng, H. Polymeric organo–magnesium complex for room temperature hydrogen physisorption. RSC Adv. 2015, 5, 10886–10891. [Google Scholar] [CrossRef]
- Kessler, F.K.; Schuhbeck, A.M.; Schnick, W. Melamium Thiocyanate Melam, a Melamium Salt with Disordered Anion Sites. Z. Anorg. Allg. Chem. 2019, 645, 840–847. [Google Scholar] [CrossRef]
- Sattler, A.; Schnick, W. Preparation and Structure of Melemium Melem Perchlorate HC6N7(NH2)3ClO4·C6N7(NH2)3. Z. Anorg. Allg. Chem. 2008, 634, 457–460. [Google Scholar] [CrossRef]
- Sattler, A.; Schnick, W. Melemium Hydrogensulfate H3C6N7(NH2)3(HSO4)3—The First Triple Protonation of Melem. Z. Anorg. Allg. Chem. 2010, 636, 2589–2594. [Google Scholar] [CrossRef]
- Vella-Zarb, L.; Braga, D.; Guy Orpen, A.; Baisch, U. The influence of hydrogen bonding on the planar arrangement of melamine in crystal structures of its solvates, cocrystals and salts. CrystEngComm 2014, 16, 8147–8159. [Google Scholar] [CrossRef]
- Kessler, F.K.; Koller, T.J.; Schnick, W. Synthesis and Structure of Melamium Bromide C6N11H10Br and Melamium Iodide C6N11H10I. Z. Anorg. Allg. Chem. 2018, 644, 186–192. [Google Scholar] [CrossRef]
- Braml, J.; Perpétuo, G.J. Bis (melaminium) sulfate dihydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2001, 57, 1431–1433. [Google Scholar] [CrossRef]
- Volfkovi, S.I.; Feldmann, W.W.; Kozmina, M.L. Über Kondensierte Phosphate des Melamins. Z. Anorg. Allg. Chem. 1979, 457, 20–30. [Google Scholar] [CrossRef]
- Janczak, J.; Perpétuo, G.J. Melaminium chloride hemihydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2001, 57, 1120–1122. [Google Scholar] [CrossRef] [PubMed]
- Janczak, J.; Perpétuo, G.J. Melaminium phthalate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2001, 57, 123–125. [Google Scholar] [CrossRef]
- Marchewka, M.; Pietraszko, A. Structure and spectra of melaminium citrate. J. Phys. Chem. Solids 2003, 64, 2169–2181. [Google Scholar] [CrossRef]
- Colombo, A.; Menabue, L.; Motori, A.; Pellacani, G.C.; Porzio, W.; Sandrolini, F.; Willett, R. Crystal structure and spectroscopic, magnetic, and electrical properties of a copper (II) dimer, melaminium hexachlorodicuprate, exhibiting a new stacking interaction. Inorg. Chem. 1985, 24, 2900–2905. [Google Scholar] [CrossRef]
- Kroenke, W.J.; Fackler, J.P., Jr.; Mazany, A.M. Structure and bonding of melaminium. beta-octamolybdate. Inorg. Chem. 1983, 22, 2412–2416. [Google Scholar] [CrossRef]
- Sattler, A.; Seyfarth, L.; Senker, J.; Schnick, W. Synthesen, Kristallstrukturen und spektroskopische Eigenschaften des Melem-Adduktes C6N7(NH2)3·H3PO4 sowie der Melemium-Salze (H2C6N7(NH2)3)SO4·2H2O und (HC6N7(NH2)3)ClO4·H2O. Z. Anorg. Allg. Chem. 2005, 631, 2545–2554. [Google Scholar] [CrossRef]
- Sattler, A.; Schönberger, S.; Schnick, W. Melemium Methylsulfonates HC6N7(NH2)3H2C6N7(NH2)3(SO3Me)3·H2O and H2C6N7(NH2)3(SO3Me)2·H2O. Z. Anorg. Allg. Chem. 2010, 636, 475–482. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Li, Z.-J.; Cheng, J.-K.; Yin, P.-X.; Yao, Y.-G. New Coordination Motifs of Melamine Directed by N−H···X (X=Cl or Br) Hydrogen Bonds. Inorg. Chem. 2007, 46, 5838–5840. [Google Scholar] [CrossRef]
- Rana, A.; Bera, M.; Chowdhuri, D.S.; Hazari, D.; Jana, S.K.; Zangrando, E.; Dalai, S. 3D Coordination Network of Ag(I) Ions with μ3 -Bridging Melamine Ligands. J. Inorg. Organomet. Polym. Mater. 2012, 22, 360–368. [Google Scholar] [CrossRef]
- Bai, Z.; Lee, J.; Kim, H.; Hu, C.L.; Ok, K.M. Unveiling the Superior Optical Properties of Novel Melamine-Based Nonlinear Optical Material with Strong Second-Harmonic Generation and Giant Optical Anisotropy. Small, 2023; in press. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, Y.; Ma, L.; Fan, G.; Gao, W.; Wang, W.; Ma, X. A new melamine-based Cu(I) coordination polymer with an excellent photocatalytic activity, therapeutic and nursing effects on the blood glucose regulation. J. Struct. Chem. 2022, 63, 302–309. [Google Scholar] [CrossRef]
- Braml, N.E.; Sattler, A.; Schnick, W. Formation of melamium adducts by pyrolysis of thiourea or melamine/NH4Cl mixtures. Chem.-Eur. J. 2012, 18, 1811–1819. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Ren, J. Gas-phase acid-base properties of melamine and cyanuric acid. J. Am. Soc. Mass Spectrom. 2010, 21, 1720–1729. [Google Scholar] [CrossRef]
- Gorne, A.L.; Scholz, T.; Kobertz, D.; Dronskowski, R. Deprotonating Melamine to Gain Highly Interconnected Materials: Melaminate Salts of Potassium and Rubidium. Inorg. Chem. 2021, 60, 15069–15077. [Google Scholar] [CrossRef]
- Gorne, A.L.; George, J.; van Leusen, J.; Duck, G.; Jacobs, P.; Chogondahalli Muniraju, N.K.; Dronskowski, R. Ammonothermal Synthesis, Crystal Structure, and Properties of the Ytterbium(II) and Ytterbium(III) Amides and the First Two Rare-Earth-Metal Guanidinates, YbC(NH)3 and Yb(CN3H4)3. Inorg. Chem. 2016, 55, 6161–6168. [Google Scholar] [CrossRef]
- Franklin, E.C. The ammono carbonic acids. J. Am. Chem. Soc. 1922, 44, 486–509. [Google Scholar] [CrossRef]
- Schnick, W.; Huppertz, H. Darstellung, Kristallstruktur und Eigenschaften von Kaliumhydrogencyanamid. Z. Anorg. Allg. Chem. 1995, 621, 1703–1707. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physic, 95th ed.; CRC Press LLC: Boca Raton, FL, USA, 2016. [Google Scholar]
- Putz, H.; Brandenburg, K. Match!—Phase Analysis Using Powder Diffraction, Version 3.15.x; Crystal Impact: Bonn, Germany, 2023. [Google Scholar]
- Sheldrick, G.M. SHELXS-97 and SHELXL-97, Program for Crystal Structure Solution and Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Dolomanov, O.; Bourhis, L.; Gildea, R.; Howard, J.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Mos, A.; Castro, C.; Indris, S.; Ströbele, M.; Fink, R.F.; Meyer, H.-J. From WCl6 to WCl2: Properties of Intermediate Fe–W–Cl Phases. Inorg. Chem. 2015, 54, 9826–9832. [Google Scholar] [CrossRef]
- Ströbele, M.; Mos, A.; Meyer, H.-J. Cluster harvesting by successive reduction of a metal halide with a nonconventional reduction agent: A benefit for the exploration of metal-rich halide systems. Inorg. Chem. 2013, 52, 6951–6956. [Google Scholar] [CrossRef] [PubMed]
- Ströbele, M.; Meyer, H.-J. Pandora’s box of binary tungsten iodides. Dalton Trans. 2019, 48, 1547–1561. [Google Scholar] [CrossRef]
- Lizarazo-Jaimes, E.H.; Reis, P.G.; Bezerra, F.M.; Rodrigues, B.L.; Monte-Neto, R.L.; Melo, M.N.; Frezard, F.; Demicheli, C. Complexes of different nitrogen donor heterocyclic ligands with SbCl3 and PhSbCl2 as potential antileishmanial agents against Sb(III)-sensitive and -resistant parasites. J. Inorg. Biochem. 2014, 132, 30–36. [Google Scholar] [CrossRef]
- Kalmutzki, M.; Ströbele, M.; Bettinger, H.F.; Meyer, H.-J. Development of Metal Cyanurates: The Example of Barium Cyanurate (BCY). Eur. J. Inorg. Chem. 2014, 2014, 2536–2543. [Google Scholar] [CrossRef]
- Kalmutzki, M.; Ströbele, M.; Enseling, D.; Jüstel, T.; Meyer, H.-J. Synthesis, Structure, and Luminescence of Rare Earth Cyanurates. Eur. J. Inorg. Chem. 2015, 2015, 134–140. [Google Scholar] [CrossRef]
- Salah, T.; Mhadhbi, N.; Ben Ahmed, A.; Hamdi, B.; Krayem, N.; Loukil, M.; Guesmi, A.; Khezami, L.; Houas, A.; Ben Hamadi, N. Physico-Chemical Characterization, DFT Modeling and Biological Activities of a New Zn(II) Complex Containing Melamine as a Template. Crystals 2023, 13, 746. [Google Scholar] [CrossRef]
- Li, F.; Xu, H.; Xu, X.; Cang, H.; Xu, J.; Chen, S. Supramolecular salts assembled by melamine and two organic hydroxyl acids: Synthesis, structure, hydrogen bonds, and luminescent property. CrystEngComm 2021, 23, 2235–2248. [Google Scholar] [CrossRef]
- Mitra, M.; Hossain, A.; Manna, P.; Choudhury, S.R.; Kaenket, S.; Helliwell, M.; Bauzá, A.; Frontera, A.; Mukhopadhyay, S. Melamine-mediated self-assembly of a Cu(II)–methylmalonate complex assisted by π+–π+ and anti-electrostatic H-bonding interactions. J. Coord. Chem. 2017, 70, 463–474. [Google Scholar] [CrossRef]
- Araar, H.; Benounis, M.; Direm, A.; Touati, A.; Atailia, S.; Barhoumi, H.; Jaffrezic-Renault, N. A new thin film modified glassy carbon electrode based on melaminium chloride pentachlorocuprate (II) for selective determination of nitrate in water. Mon. Chem. 2019, 150, 1737–1744. [Google Scholar] [CrossRef]
- Hesse, M.; Meier, H.; Zeeh, B. Spektroskopische Methoden in der Organischen Chemie; Georg Thieme: Stuttgart, Germany, 2005. [Google Scholar]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy; Cengage Learning: Boston, MA, USA, 2014; pp. 14–101. [Google Scholar]
- Lotsch, B.V.; Döblinger, M.; Sehnert, J.; Seyfarth, L.; Senker, J.; Oeckler, O.; Schnick, W. Unmasking melon by a complementary approach employing electron diffraction, solid-state NMR spectroscopy, and theoretical calculations—Structural characterization of a carbon nitride polymer. Chem.-Eur. J. 2007, 13, 4969–4980. [Google Scholar] [CrossRef]






| Compound | (1) | (2) | (3) |
|---|---|---|---|
| CCDC code | 2201273 | 2210244 | 2213381 |
| Formula weight | 642.97 | 1033.67 | 281.32 |
| Temperature/K | 220.0(1) | 150.0(1) | 150.0(1) |
| Wavelength/Å | 1.54184 | 1.54184 | 1.54184 |
| Space group | P | P21/c | P21/n |
| a/Å | 9.5878(5) | 13.2780(2) | 5.3562(2) |
| b/Å | 10.5395(3) | 10.6878(1) | 10.5432(3) |
| c/Å | 11.4338(5) | 24.0953(2) | 12.5618(4) |
| α/° | 74.011(3) | 90 | 90 |
| β/° | 79.122(4) | 105.860(1) | 93.710(3) |
| γ/° | 85.602(3) | 90 | 90 |
| Volume/Å3 | 1090.37(8) | 3289.26(7) | 707.90(4) |
| Z | 2 | 4 | 4 |
| Rint | 0.0364 | 0.0485 | 0.0312 |
| Goodness-of-fit on F2 | 1.074 | 1.044 | 1.044 |
| wR2 (all data) | 0.0660 | 0.0607 | 0.0267 |
| wR2 | 0.0643 | 0.0603 | 0.0264 |
| Final R indices (all data) | 0.0339 | 0.0251 | 0.0120 |
| R1 | 0.0278 | 0.0243 | 0.0110 |
| ϴMax./° | 4.365 | 3.460 | 5.483 |
| ϴMin./° | 66.585 | 66.583 | 70.067 |
| μ/mm−1 | 14.93 | 19.478 | 33.932 |
| ΔρMax./e·Å−3 | 0.508 | 2.491 | 0.322 |
| ΔρMin./e·Å−3 | −0.593 | −0.597 | −0.451 |
| Completeness/% | 97.3 | 100 | 99.8 |
| Compound (1) | Compound (2) | Compound (3) | ||||||
|---|---|---|---|---|---|---|---|---|
| Atom | Atom | Length/pm | Atom | Atom | Length/pm | Atom | Atom | Length/pm |
| Sb1 | Cl1 | 276.5(8) | Sb1 | Cl3 | 284.7(6) | Sb1 | N2 | 241.5(1) |
| Sb1 | N1 | 253.6(3) | Sb1 | Cl4 | 260.6(0) | Sb1 | N6 | 208.6(8) |
| Sb1 | Cl2 | 247.0(0) | Sb1 | Cl5 | 248.7(2) | Sb1 | N4 | 204.4(6) |
| Sb1 | Cl3 | 256.8(2) | Sb1 | Cl1 | 240.2(1) | Sb1 | Cl1 | 254.9(3) |
| Sb1 | N4 | 204.7(3) | Sb2 | N1 | 256.1(3) | |||
| Sb2 | N4 | 204.7(3) | ||||||
| Sb2 | Cl6 | 279.2(7) | ||||||
| Sb2 | Cl7 | 247.2(8) | ||||||
| Sb2 | Cl8 | 251.1(1) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayat, E.; Ströbele, M.; Meyer, H.-J. Unraveling the Synthesis of SbCl(C3N6H4): A Metal-Melaminate Obtained through Deprotonation of Melamine with Antimony(III)Chloride. Chemistry 2023, 5, 1465-1476. https://doi.org/10.3390/chemistry5020099
Bayat E, Ströbele M, Meyer H-J. Unraveling the Synthesis of SbCl(C3N6H4): A Metal-Melaminate Obtained through Deprotonation of Melamine with Antimony(III)Chloride. Chemistry. 2023; 5(2):1465-1476. https://doi.org/10.3390/chemistry5020099
Chicago/Turabian StyleBayat, Elaheh, Markus Ströbele, and Hans-Jürgen Meyer. 2023. "Unraveling the Synthesis of SbCl(C3N6H4): A Metal-Melaminate Obtained through Deprotonation of Melamine with Antimony(III)Chloride" Chemistry 5, no. 2: 1465-1476. https://doi.org/10.3390/chemistry5020099