Catalytic Reductive Amination of Aromatic Aldehydes on Co-Containing Composites
Abstract
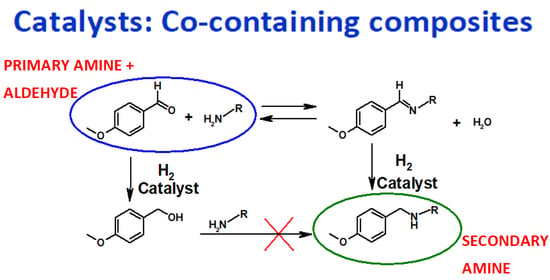
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Co-Im/SiO2 Composite
3.2. General Procedure for the Amination
3.3. NMR Data of the Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afanasyev, O.I.; Kuchuk, E.; Usanov, D.L.; Chusov, D. Reductive amination in the synthesis of pharmaceuticals. Chem. Rev. 2019, 119, 11857–11911. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Azuma, K.; Yamano, M. Highly enantioselective direct asymmetric reductive amination of 2-acetyl-6-substituted pyridines. Org. Lett. 2021, 23, 3364–3367. [Google Scholar] [CrossRef] [PubMed]
- Roughley, S.D.; Jordan, A.M. The medicinal chemist’s toolbox: An analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 2011, 54, 3451–3479. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Magid, A.F.; Mehrman, S.J. A review on the use of sodium triacetoxyborohydride in the reductive amination of ketones and aldehydes. Org. Process Res. Dev. 2006, 10, 971–1031. [Google Scholar] [CrossRef]
- Dangerfield, E.M.; Plunkett, C.H.; Win-Mason, A.L.; Stocker, B.L.; Timmer, M.S.M. Protecting-group-free synthesis of amines: Synthesis of primary amines from aldehydes via reductive amination. J. Org. Chem. 2010, 75, 5470–5477. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.W.; Cho, Y.J.; Lee, S.H.; Yoon, C.-O.M.; Yoon, C.M. A one-pot synthesis of N-alkylaminobenzenes from nitroaromatics: Reduction followed by reductive amination using B10H14. Chem. Commun. 2000, 19, 1857–1858. [Google Scholar] [CrossRef]
- Zou, Q.; Liu, F.; Zhao, T.; Hu, X. Reductive amination of ketones/aldehydes with amines using BH3N(C2H5)3 as a reductant. Chem. Commun. 2021, 57, 8588–8591. [Google Scholar] [CrossRef]
- Lee, O.Y.; Law, K.L.; Ho, C.Y.; Yang, D. Highly chemoselective reductive amination of carbonyl compounds promoted by InCl3/Et3SiH/MeOH system. J. Org. Chem. 2008, 73, 8829–8837. [Google Scholar] [CrossRef]
- Li, B.; Zheng, J.; Zeng, W.; Li, Y.; Chen, L. Efficient Ruthenium(II)-catalyzed direct reductive amination of aldehydes under mild conditions using hydrosilane as the reductant. Synthesis 2017, 49, 1349–1355. [Google Scholar] [CrossRef] [Green Version]
- Nayal, O.S.; Bhatt, V.; Sharma, S.; Kumar, N. Chemoselective reductive amination of carbonyl compounds for the synthesis of tertiary amines using SnCl2·2H2O/PMHS/MeOH. J. Org. Chem. 2015, 80, 5912–5918. [Google Scholar] [CrossRef]
- Apodaca, R.; Xiao, W. Direct reductive amination of aldehydes and ketones using phenylsilane: Catalysis by dibutyltin dichloride. Org. Lett. 2001, 3, 1745–1748. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Ouyang, L.; Liao, J.; Yang, X.; Luo, R. Construction of α-alkylated amines by Iridium complex-catalyzed one-step transfer hydrogenation of C=C and C=N bonds. Synthesis 2021, 53, 1821–1827. [Google Scholar] [CrossRef]
- Alonso, F.; Riente, P.; Yus, M. Hydrogen-transfer reductive amination of aldehydes catalysed by Nickel nanoparticles. Synlett 2008, 9, 1289–1292. [Google Scholar] [CrossRef]
- Mignonac, G. Nouvelle Méthode Générale de Préparation des Amines à Partir des Aldéhydes ou des Cétones. Comptes Rendus 1921, 172, 223–226. Available online: https://gallica.bnf.fr/ark:/12148/bpt6k3125x/f37.item (accessed on 30 December 2022).
- Podyacheva, E.; Afanasyev, O.I.; Tsygankov, A.A.; Makarova, M.; Chusov, D. Hitchhiker’s guide to reductive amination. Synthesis 2019, 51, 2667–2677. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, R.P.; Verma, S.S.; Pandey, J.; Tiwari, V. Recent development on catalytic reductive amination and applications. Curr. Org. Chem. 2008, 12, 1093–1115. [Google Scholar] [CrossRef]
- Nugent, T.C.; El-Shazly, M. Chiral amine synthesis—Recent developments and trends for enamide reduction, reductive amination, and imine reduction. Adv. Synth. Catal. 2010, 352, 753–819. [Google Scholar] [CrossRef]
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 6th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Hong, Z.; Ge, X.; Zhou, S. Underlying mechanisms of reductive amination on Pd-catalysts: The unique role of hydroxyl group in generating sterically hindered amine. Int. J. Mol. Sci. 2022, 23, 7621. [Google Scholar] [CrossRef]
- Ge, X.; Pan, J.; Qian, C.; Feng, L.; Chen, Y.; Chen, X. Mechanism study on Raney nickel-catalyzed amination of resorcinol. Catal. Commun. 2014, 46, 201–207. [Google Scholar] [CrossRef]
- Truong, C.C.; Mishra, D.K.; Suh, Y.-W. Recent catalytic advances on the sustainable production of primary furanic amines from the one-pot reductive amination of 5-hydroxymethylfurfural. ChemSusChem, 2022; in press. [Google Scholar] [CrossRef]
- CH Guideline Q3D (R2) on Elemental Impurities, EMA/CHMP/ICH/353369/2013, Committee for Medicinal Products for Human Use, European Medicines Agency, Amsterdam, The Netherlands. First Published 03.05.2022, Legal Affective Date 24.09.2022. Available online: https://www.ema.europa.eu/en/ich-q3d-elemental-impurities-scientific-guideline (accessed on 30 December 2022).
- Hahn, G.; Kunnas, P.; de Jonge, N.; Kempe, R. General synthesis of primary amines via reductive amination employing a reusable nickel catalyst. Nat. Catal. 2019, 2, 71–77. [Google Scholar] [CrossRef]
- Murugesan, K.; Beller, M.; Jagadeesh, R.V. Reusable nickel nanoparticles-catalyzed reductive amination for selective synthesis of primary amines. Angew. Chem. Int. Ed. 2019, 131, 5118–5122. [Google Scholar] [CrossRef]
- Gould, N.S.; Landfield, H.; Dinkelacker, B.; Brady, C.; Yang, X.; Xu, B. Selectivity control in catalytic reductive amination of furfural to furfurylamine on supported catalysts. ChemCatChem 2020, 12, 2106–2115. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, H.; Shu, H.; Xiao, S.; Guo, D.; Liu, Y.; Wei, Z.; Li, X. A comprehensive study on the reductive amination of 5-hydroxymethylfurfural into 2,5-bisaminomethylfuran over Raney Ni through DFT calculations. ChemCatChem 2019, 11, 2649–2656. [Google Scholar] [CrossRef]
- Wei, Z.; Cheng, Y.; Zhou, K.; Zeng, Y.; Yao, E.; Li, Q.; Liu, Y.; Sun, Y. One-step reductive amination of 5-hydroxymethylfurfural into 2,5-bis(aminomethyl)furan over Raney Ni. ChemSusChem 2021, 14, 2308–2312. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Cheng, Y.; Huang, H.; Ma, Z.; Zhou, K.; Liu, Y. Reductive amination of 5-hydroxymethylfurfural to 2,5-bis(aminomethyl)furan over alumina-supported Ni-based catalytic systems. ChemSusChem 2022, 15, e202200233. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesh, R.V.; Murugesan, K.; Alshammari, A.S.; Neumann, H.; Pohl, M.M.; Radnik, J.; Beller, M. MOF-derived cobalt nanoparticles catalyze a general synthesis of amines. Science 2017, 358, 326–332. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Chen, B.; Zhou, X.; Kang, S.; Xu, Y.; Wei, J. Selective synthesis of furfurylamine by reductive amination of furfural over Raney cobalt. ChemCatChem 2019, 11, 5562–5569. [Google Scholar] [CrossRef]
- Pisiewicz, S.; Stemmler, T.; Surkus, A.-E.; Junge, K.; Beller, M. Synthesis of amines by reductive amination of aldehydes and ketones using Co3O4/NGr@C catalyst. ChemCatChem 2015, 7, 62–64. [Google Scholar] [CrossRef]
- Mao, F.; Sui, D.; Qi, Z. Heterogeneous cobalt catalysts for reductive amination with H2: General synthesis of secondary and tertiary amines. RSC Adv. 2016, 6, 94068–94073. [Google Scholar] [CrossRef]
- Bäumler, C.; Bauer, C.; Kempe, R. The synthesis of primary amines via reductive amination employing an iron catalyst. ChemSusChem 2020, 13, 3110–3114. [Google Scholar] [CrossRef]
- Cabrero-Antonino, J.R.; Adam, R.; Papa, V.; Beller, M. Homogeneous and heterogeneous catalytic reduction of amides and related compounds using molecular hydrogen. Nat. Commun. 2020, 11, 3893. [Google Scholar] [CrossRef] [PubMed]
- Timelthaler, D.; Topf, C. Heterogeneous Hydrogenation of Quinoline Derivatives Effected by a Granular Cobalt Catalyst. Synthesis 2022, 54, 629–642. [Google Scholar] [CrossRef]
- Subotin, V.V.; Vashchenko, B.V.; Asaula, V.M.; Verner, E.V.; Ivanytsya, M.O.; Shvets, O.; Ostapchuk, E.N.; Grygorenko, O.O.; Ryabukhin, S.V.; Volochnyuk, D.M.; et al. Screening of Palladium/Charcoal Catalysts for Hydrogenation of Diene Carboxylates with Isolated-Rings (Hetero)aliphatic Scaffold. Molecules 2023, 28, 1201. [Google Scholar] [CrossRef] [PubMed]
- Baidilov, D.; Hayrapetyan, D.; Khalimon, A.Y. Recent advances in homogeneous base-metal-catalyzed transfer hydrogenation reactions. Tetrahedron 2021, 98, 132435. [Google Scholar] [CrossRef]
- Facchini, S.V.; Cettolin, M.; Bai, X.; Casamassima, G.; Pignataro, L.; Gennari, C.; Piarulli, U. Efficient Synthesis of Amines by Iron-Catalyzed C=N Transfer Hydrogenation and C=O Reductive Amination. Adv. Synth. Catal. 2018, 360, 1054–1059. [Google Scholar] [CrossRef]
- Dong, M.; Gao, X.; Xiang, Y.; Li, L.; Li, S.; Wang, X.; Li, Z.; Zhu, H. Highly enantioselective transfer hydrogenation catalyzed by diasteromeric mixtures of axially chiral (aR,S)- and (aS,S)-Biscarbolines. Tetrahedron 2021, 82, 131924. [Google Scholar] [CrossRef]
- Ouyang, L.; Xia, Y.; Liao, J.; Luo, R. One-Pot Transfer Hydrogenation Reductive Amination of Aldehydes and Ketones by Iridium Complexes “on Water”. Eur. J. Org. Chem. 2020, 2020, 6387–6391. [Google Scholar] [CrossRef]
- Westerhaus, F.A.; Jagadeesh, R.V.; Wienhöfer, G.; Pohl, M.-M.; Radnik, J.; Surkus, A.-E.; Rabeah, J.; Junge, K.; Junge, H.; Nielsen, M.; et al. Heterogenized cobalt oxide catalysts for nitroarene reduction by pyrolysis of molecularly defined complexes. Nat. Chem. 2013, 5, 537–543. [Google Scholar] [CrossRef]
- Chen, F.; Surkus, A.-E.; He, L.; Pohl, M.-M.; Radnik, J.; Topf, C.; Junge, K.; Beller, M. Selective catalytic hydrogenation of heteroarenes with N-graphene-modified cobalt nanoparticles (Co3O4–Co/NGr@α-Al2O3). J. Am. Chem. Soc. 2015, 137, 11718–11724. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Surkus, A.-E.; Junge, H.; Pohl, M.-M.; Radnik, J.; Rabeah, J.; Huan, H.; Schünemann, V.; Brückner, A.; Beller, M. Nanoscale Fe2O3-based catalysts for selective hydrogenation of nitroarenes to anilines. Science 2013, 342, 1073–1076. [Google Scholar] [CrossRef]
- Asaula, V.M.; Shvets, O.V.; Pariiska, O.O.; Bur’yanov, V.V.; Ryabukhin, S.V.; Volochnyuk, D.M.; Kolotilov, S.V. Composites based on nanodispersed nickel, graphene-like carbon, and aerosil for catalytic hydrogenation of furfural and quinoline. Theor. Exp. Chem. 2020, 56, 261–267. [Google Scholar] [CrossRef]
- Asaula, V.M.; Buryanov, V.V.; Solod, B.Y.; Tryus, D.M.; Pariiska, O.O.; Kotenko, I.E.; Volovenko, Y.M.; Volochnyuk, D.M.; Ryabukhin, S.V.; Kolotilov, S.V. Catalytic hydrogenation of substituted quinolines on Co-graphene composites. Eur. J. Org. Chem. 2021, 2021, 6623–6632. [Google Scholar] [CrossRef]
- Lee, J.S.; Wang, X.; Luo, H.; Baker, G.A.; Dai, S. Facile Ionothermal Synthesis of Microporous and Mesoporous Carbons from Task Specific Ionic Liquids. J. Am. Chem. Soc. 2009, 131, 4596–4597. [Google Scholar] [CrossRef] [PubMed]
- Leicester, J.; Redman, M.J. Thermal decomposition of the nickel and cobalt salts of aliphatic acids. J. Appl. Chem. 1962, 12, 357–366. [Google Scholar] [CrossRef]
- Pels, J.R.; Kapteijn, F.; Moulijn, J.A.; Zhu, Q.; Thomas, K.M. Evolution of nitrogen functionalities in carbonaceous materials during pyrolysis. Carbon 1995, 33, 1641–1653. [Google Scholar] [CrossRef]
- Owen, E.A.; Madoc Jones, D. Effect of grain size on the crystal structure of cobalt. Proc. Phys. Soc. B 1954, 67, 456–466. [Google Scholar] [CrossRef]
- Kotbagi, T.V.; Gurav, H.R.; Nagpure, A.S.; Chilukuri, S.V.; Bakker, M.G. Highly efficient nitrogen-doped hierarchically porous carbon supported Ni nanoparticles for the selective hydrogenation of furfural to furfuryl alcohol. RSC Adv. 2016, 6, 67662–67668. [Google Scholar] [CrossRef] [Green Version]
- Qiao, X.; Jin, J.; Fan, H.; Cui, L.; Ji, S.; Li, Y.; Liao, S. Cobalt and Nitrogen co-doped Graphene-Carbon nanotube aerogel as an efficient bifunctional electrocatalyst for oxygen reduction and evolution reactions. Catalysts 2018, 8, 275. [Google Scholar] [CrossRef] [Green Version]
- Ni, Z.; Wang, Y.; Yu, T.; Shen, Z. Raman Spectroscopy and Imaging of Graphene. Nano Res. 2008, 1, 273–291. [Google Scholar] [CrossRef] [Green Version]
- Yoon, D.; Cheong, H. Raman Spectroscopy for Characterization of Graphene. In Raman Spectroscopy for Nanomaterials Characterization; Kumar, C.S.S.R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Das, A.; Chakraborty, B.; Sood, A.K. Raman spectroscopy of graphene on different substrates and influence of defects. Bull. Mater. Sci. 2008, 31, 579–584. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C. Purification of Laboratory Chemicals; Elsevier: Oxford, UK, 2003. [Google Scholar]






| Composite | Organic Precursor for Pyrolysis | ω(Co) in the Composite, % by Weight | Ref. |
|---|---|---|---|
| Co-Im/SiO2 | imidazole | 5.7 | this work |
| Co-Phen/SiO2 (a) | 1,10-phenantroline | 3.7 | [45] |
| Co-DAB/SiO2-1 (b) | 1,2-diaminobenzene | 8.0 | [45] |
| Co-DAB/SiO2-2 (c) | 1,2-diaminobenzene | 5.3 | [45] |
| Co-Mel/SiO2 (d) | melamine | 3.4 | [45] |
| Product Yields for n-Butylamine (1.0 mmol) % (a) | Product Yields for n-Butylamine (1.5 mmol) % (a) | Product Yields for Benzylamine (1 mmol) % (a) | Product Yields for Diisopropylamine, (1.5 mmol), % (a) | Yield of THQ (b), % | ||
|---|---|---|---|---|---|---|
| P, bar | 100 | 150 | 100 | 100 | 100 | 100 |
| T, °C | 50 | 150 | 100 | 100 | 100 | 100 |
| Co loading, mol.% | 2 | 5 | 3 | 3 | 3 | 3 |
| Co-Im/SiO2 | SC 100 | AM 100 | AM 92 AL 8 | AM 92 AL 8 | AL 90 ALD 10 | 55 |
| Co-Phen/SiO2-1 | SC 100 | AM 100 | AM 86 AL 2 SC 12 | AM 92 AL 6 SC 2 | AL 90 ALD 10 | 13 |
| Co-DAB/SiO2-1 | SC 100 | AM 100 | AM 88 AL 12 | AM 92 AL 8 | AL 99 | 14 |
| Co-DAB/SiO2-2 | SC 100 | AM 100 | AM 94 AL 6 | AM 96 AL 4 | AL 99 | 32 |
| Co-Mel/SiO2-4 | SC 100 | AM 100 | AM 72 AL 19 SC 9 | AM 73 AL 27 | AL 99 | 16 |
| Co-Mel/SiO2-4 (c) | AM 89% AL 3% SC 8% | AM 85% AL 4% SC 11% | AL 99 | |||
| Catalyst | Product Yields % (a) |
|---|---|
| Co-Im/SiO2 | Cl-AM 60; Cl-Al 0.5; ET 33; Cl-ET 1 |
| Co-Phen/SiO2-1 | Cl-AM 68; Cl-AL 4; Cl-ET 17 |
| Co-DAB/SiO2-1 | Cl-AM 89; Cl-AL 0.5; Cl-ET 7 |
| Co-DAB/SiO2-2 | Cl-AM 74; Cl-AL 1; AM 2.5; Cl-ET 13 |
| Co-Mel/SiO2-4 | Cl-AM 70; Cl-AL 7; Cl-ET 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subotin, V.V.; Asaula, V.M.; Lishchenko, Y.L.; Ivanytsya, M.O.; Pariiska, O.O.; Ryabukhin, S.V.; Volochnyuk, D.M.; Kolotilov, S.V. Catalytic Reductive Amination of Aromatic Aldehydes on Co-Containing Composites. Chemistry 2023, 5, 281-293. https://doi.org/10.3390/chemistry5010022
Subotin VV, Asaula VM, Lishchenko YL, Ivanytsya MO, Pariiska OO, Ryabukhin SV, Volochnyuk DM, Kolotilov SV. Catalytic Reductive Amination of Aromatic Aldehydes on Co-Containing Composites. Chemistry. 2023; 5(1):281-293. https://doi.org/10.3390/chemistry5010022
Chicago/Turabian StyleSubotin, Vladyslav V., Vitalii M. Asaula, Yulian L. Lishchenko, Mykyta O. Ivanytsya, Olena O. Pariiska, Sergey V. Ryabukhin, Dmitriy M. Volochnyuk, and Sergey V. Kolotilov. 2023. "Catalytic Reductive Amination of Aromatic Aldehydes on Co-Containing Composites" Chemistry 5, no. 1: 281-293. https://doi.org/10.3390/chemistry5010022