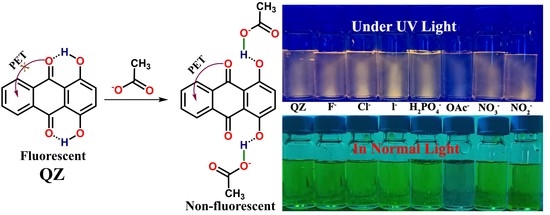
Selective Recognition and Reversible “Turn-Off” Fluorescence Sensing of Acetate (CH3COO−) Anion at Ppb Level Using a Simple Quinizarin Fluorescent Dye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentations
2.3. Fluorescence Sensing Studies
3. Results and Discussion
3.1. Anion Sensing Studies Using UV–Vis Titrations
3.2. Anion Sensing Using Fluorescence Titrations Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goshisht, M.K.; Tripathi, N. Fluorescence-based Sensors as an Emerging Tool for Anion Detection: Mechanism, Sensory materials, and Applications. J. Mater. Chem. C 2021, 9, 9820–9850. [Google Scholar] [CrossRef]
- Pal, A.; Karmakar, M.; Bhatta, S.R.; Thakur, A. A Detailed Insight into Anion Sensing based on Intramolecular Charge Transfer (ICT) Mechanism: A Comprehensive Review of the Years 2016 to 2021. Coord. Chem. Rev. 2021, 448, 214167. [Google Scholar] [CrossRef]
- Ali, R.; Razi, S.S.; Gupta, R.C.; Dwivedi, S.K.; Misra, A. An Efficient ICT-based Fluorescent Turn-on Dyad for Selective Detection of Fluoride and Carbon dioxide. New J. Chem. 2016, 40, 162–170. [Google Scholar] [CrossRef]
- De Azevedo, L.C.; Reis, M.M.; Motta, L.F.; da Rocha, G.O.; Silva, L.A.; de Andrade, J.B. Evaluation of the Formation and Stability of Hydroxyalkylsulfonic Acids in Wines. J. Agric. Food Chem. 2007, 55, 8670–8680. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cooper, D.E.; Cluntun, A.A.; Warmoes, M.O.; Zhao, S.; Reid, M.A.; Liu, J.; Lund, P.J.; Lopes, M.; Garcia, B.A. Acetate Production from Glucose and Coupling to Mitochondrial Metabolism in Mammals. Cell 2018, 175, 502–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauer, H.; Wartenberg, M.; Hescheler, J. Reactive Oxygen Species as Intracellular Messengers during Cell Growth and Differentiation. Cell. Physiol. Biochem. 2001, 11, 173–186. [Google Scholar] [CrossRef]
- Bowman-James, K.; Bianchi, A.; García-España, E. Anion Coordination Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 3527639519. [Google Scholar]
- De Silva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling Recognition Events with Fluorescent Sensors and Switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef] [PubMed]
- Beer, P.D.; Gale, P.A. Anion Recognition and Sensing: The State of the Art and Future Perspectives. Angew. Chem. Int. Ed. 2001, 40, 486–516. [Google Scholar] [CrossRef]
- Martinez-Manez, R.; Sancenon, F. Fluorogenic, and Chromogenic Chemosensors and Reagents for Anions. Chem. Rev. 2003, 103, 4419–4476. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, X.; Kim, H.N.; Yoon, J. Sensors for the Optical Detection of Cyanide Ion. Chem. Soc. Rev. 2010, 39, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Y.; Zhang, X.; Zhang, S.; Zhang, Z. A New Coumarin Based Chromo-Fluorogenic Probe for Selective Recognition of Cyanide Ions in an Aqueous Medium. RSC Adv. 2015, 5, 96905–96910. [Google Scholar] [CrossRef]
- Ho, T.Y.; Scranton, M.I.; Taylor, G.T.; Varela, R.; Thunell, R.C.; Muller-Karger, F. Acetate Cycling in the Water Column of the Cariaco Basin: Seasonal and Vertical Variability and Implication for Carbon Cycling. Limnol. Oceanogr. 2002, 47, 1119–1128. [Google Scholar] [CrossRef]
- Orzeł, Ł.; Fiedor, L.; Wolak, M.; Kania, A.; van Eldik, R.; Stochel, G. Interplay between Acetate Ions, Peripheral Groups, and Reactivity of the Core Nitrogens in Transmetalation of Tetrapyrroles. Chem. Eur. J. 2008, 14, 9419–9430. [Google Scholar] [CrossRef] [PubMed]
- Caltagirone, C.; Gale, P.A. Anion Receptor Chemistry: Highlights from 2007. Chem. Soc. Rev. 2009, 38, 520–563. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.H.; Cox, J.P.L.; Doig, A.J.; Gardner, M.; Gerhard, U.; Kaye, P.T.; Lal, A.R.; Nicholls, I.A.; Salter, C.J.; Mitchell, R.C. Toward the Semiquantitative Estimation of Binding Constants. Guides for Peptide-Peptide Binding in Aqueous Solution. J. Am. Chem. Soc. 1991, 113, 7020–7030. [Google Scholar] [CrossRef]
- Searle, M.S.; Williams, D.H.; Gerhard, U. Partitioning of Free Energy Contributions in the Estimation of Binding Constants: Residual Motions and Consequences for Amide-Amide Hydrogen Bond Strengths. J. Am. Chem. Soc. 1992, 114, 10697–10704. [Google Scholar] [CrossRef]
- Gunnlaugsson, T.; Glynn, M.; Tocci, G.M.; Kruger, P.E.; Pfeffer, F.M. Anion Recognition and Sensing in Organic and Aqueous Media Using Luminescent and Colorimetric Sensors. Coord. Chem. Rev. 2006, 250, 3094–3117. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Hosseini, M.; Hariri, M.; Faridbod, F.; Norouzi, P. Novel Erbium (III)-Selective Fluorimetric Bulk Optode. Sens. Actuators B Chem. 2009, 142, 90–96. [Google Scholar] [CrossRef]
- Sharma, D.; Moirangthem, A.; Sahoo, S.K.; Basu, A.; Roy, S.M.; Pati, R.K.; SK, A.K.; Nandre, J.P.; Patil, U.D. Anion Selective Chromogenic and Fluorogenic Chemosensor and Its Application in Breast Cancer Live Cell Imaging. RSC Adv. 2014, 4, 41446–41452. [Google Scholar] [CrossRef]
- Cametti, M.; Rissanen, K. Highlights on Contemporary Recognition and Sensing of Fluoride Anion in Solution and in the Solid State. Chem. Soc. Rev. 2013, 42, 2016–2038. [Google Scholar] [CrossRef] [PubMed]
- Suksai, C.; Tuntulani, T. Chromogenic Anion Sensors. Chem. Soc. Rev. 2003, 32, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Moirangthem, A.; Kumar, R.; Kumar, S.K.A.; Kuwar, A.; Callan, J.F.; Basu, A.; Sahoo, S.K. Pyridoxal-Thiosemicarbazide: Its Anion Sensing Ability and Application in Living Cells Imaging. RSC Adv. 2015, 5, 50741–50746. [Google Scholar] [CrossRef]
- Sharma, D.; Moirangthem, A.; Roy, S.M.; Kumar, A.S.K.; Nandre, J.P.; Patil, U.D.; Basu, A.; Sahoo, S.K. Bioimaging Application of a Novel Anion Selective Chemosensor Derived from Vitamin B6 Cofactor. J. Photochem. Photobiol. B Biol. 2015, 148, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.N.; Pant, P.; Sharma, D.; Sahoo, S.K.; Shankarling, G.S. Quinoline-Based Chemosensor for Fluoride and Acetate: A Combined Experimental and DFT Study. Sens. Actuators B Chem. 2014, 197, 73–80. [Google Scholar] [CrossRef]
- Chandel, M.; Roy, S.M.; Sharma, D.; Sahoo, S.K.; Patel, A.; Kumari, P.; Dhale, R.S.; Ashok, K.S.K.; Nandre, J.P.; Patil, U.D. Anion Recognition Ability of a Novel Azo Dye Derived from 4-Hydroxycoumarin. J. Lumin. 2014, 154, 515–519. [Google Scholar] [CrossRef]
- Tayade, K.; Sahoo, S.K.; Singh, A.; Singh, N.; Mahulikar, P.; Attarde, S.; Kuwar, A. Architecture of Dipodal Ratiometric Motif Showing Discrete Nanomolar Response towards Fluoride Ion. Sens. Actuators B Chem. 2014, 202, 1333–1337. [Google Scholar] [CrossRef]
- Khairnar, N.; Tayade, K.; Bothra, S.; Sahoo, S.K.; Singh, J.; Singh, N.; Bendre, R.; Kuwar, A. Novel Fluorescent Chemosensing of CN− Anions with Nanomolar Detection Using the Zn2+–Isonicotinohydrazide Metal Complex. RSC Adv. 2014, 4, 41802–41806. [Google Scholar] [CrossRef]
- Amendola, V.; Esteban-Gómez, D.; Fabbrizzi, L.; Licchelli, M. What Anions Do to N− H-Containing Receptors. Acc. Chem. Res. 2006, 39, 343–353. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Veismohammadi, B.; Hosseini, M.; Norouzi, P. A New Tb3+-Selective Fluorescent Sensor Based on 2-(5-(Dimethylamino) Naphthalene-1-Ylsulfonyl)-N-Henylhydrazinecarbothioamide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 74, 575–578. [Google Scholar] [CrossRef]
- Martínez-Máñez, R.; Sancenón, F. New Advances in Fluorogenic Anion Chemosensors. J. Fluoresc. 2005, 15, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Gunnlaugsson, T.; Ali, H.D.P.; Glynn, M.; Kruger, P.E.; Hussey, G.M.; Pfeffer, F.M.; dos Santos, C.M.G.; Tierney, J. Fluorescent Photoinduced Electron Transfer (PET) Sensors for Anions; from Design to Potential Application. J. Fluoresc. 2005, 15, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Moragues, M.E.; Martínez-Máñez, R.; Sancenón, F. Chromogenic and Fluorogenic Chemosensors and Reagents for Anions. A Comprehensive Review of the Year 2009. Chem. Soc. Rev. 2011, 40, 2593–2643. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kumar, S.K.A.; Sahoo, S.K. Vitamin B6 Cofactor Derived Chemosensor for the Selective Colorimetric Detection of Acetate Anions. Tetrahedron Lett. 2014, 55, 927–930. [Google Scholar] [CrossRef]
- Sharma, D.; Sahoo, S.K.; Chaudhary, S.; Bera, R.K.; Callan, J.F. Fluorescence ‘Turn-on’Sensor for F− Derived from Vitamin B6 Cofactor. Analyst 2013, 138, 3646–3650. [Google Scholar] [CrossRef]
- Delépine, A.-S.; Tripier, R.; Handel, H. Cyclen-Based Bismacrocycles for Biological Anion Recognition. A Potentiometric and NMR Study of AMP, ADP and ATP Nucleotide Complexation. Org. Biomol. Chem. 2008, 6, 1743–1750. [Google Scholar] [CrossRef]
- Formica, M.; Fusi, V.; Macedi, E.; Paoli, P.; Piersanti, G.; Rossi, P.; Zappia, G.; Orlando, P. New Branched Macrocyclic Ligand and Its Side-Arm, Two Urea-Based Receptors for Anions: Synthesis, Binding Studies, and Crystal Structure. New J. Chem. 2008, 32, 1204–1214. [Google Scholar] [CrossRef]
- Piątek, P.; Kalisiak, J.; Jurczak, J. Synthesis and Chiroptical Properties of Two New Planar-Chiral Macrocycles. Tetrahedron Lett. 2004, 45, 3309–3311. [Google Scholar] [CrossRef]
- Schmuck, C.; Schwegmann, M. A Molecular Flytrap for the Selective Binding of Citrate and Other Tricarboxylates in Water. J. Am. Chem. Soc. 2005, 127, 3373–3379. [Google Scholar] [CrossRef]
- Pfeffer, F.M.; Lim, K.F.; Sedgwick, K.J. Indole as a Scaffold for Anion Recognition. Org. Biomol. Chem. 2007, 5, 1795–1799. [Google Scholar] [CrossRef]
- Fisher, M.G.; Gale, P.A.; Light, M.E. A Simple Benzimidazole-Based Receptor for Barbiturate and Urea Neutral Guests That Functions in Polar Solvent Mixtures. New J. Chem. 2007, 31, 1583–1584. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, H.Y.; Lee, K.H.; Hong, J.-I. Selective Anion Sensing Based on a Dual-Chromophore Approach. Chem. Commun. 2001, 1188–1189. [Google Scholar] [CrossRef]
- Lee, D.H.; Im, J.H.; Son, S.U.; Chung, Y.K.; Hong, J.-I. An Azophenol-Based Chromogenic Pyrophosphate Sensor in Water. J. Am. Chem. Soc. 2003, 125, 7752–7753. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Luo, F.; Chen, T. Phenolphthalein Immobilized Membrane for an Optical PH Sensor. Anal. Chim. Acta 2004, 510, 189–194. [Google Scholar] [CrossRef]
- Hazneci, C.; Ertekin, K.; Yenigul, B.; Cetinkaya, E. Optical PH Sensor Based on Spectral Response of Newly Synthesized Schiff Bases. Dyes Pigment. 2004, 62, 35–41. [Google Scholar] [CrossRef]
- Guari, N.K. Anthraquinone Appended Chemosensors for Fluorescence Monitoring of Anions and/or Metal Ions. Inorg. Chim. Acta 2022, 536, 120917. [Google Scholar]
- Kaur, N.; Kumar, S. Aminoanthraquinone-based Chemosensors: Colorimetric Molecular Logic Mimicking Molecular Trafficking and a Set–Reset Memorized Device. Dalton Trans. 2012, 41, 5217–5224. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.; Kaur, N.; Kumar, S. Quinones-based Molecular Receptors for Recognition of Anions and Metal Ions. Tetrahedron 2014, 70, 4285–4307. [Google Scholar] [CrossRef]
- Shao, J. Quinoline-Based “On-Off” Fluorescent Sensor for Acetate: Effect of Link Mode between Binding Sites and Fluorophore on Fluorescence Changes. Spectrosc. Lett. 2012, 45, 262–268. [Google Scholar] [CrossRef]
- Huang, W.; Lin, H.; Lin, H. Fluorescent Acetate-Sensing in Aqueous Solution. Sens. Actuators B. 2011, 153, 404–408. [Google Scholar] [CrossRef]
- Ghosh, A.; Sengupta, A.; Chattopadhyay, A.; Das, D. A Single Probe for Sensing Both Acetate and Aluminum(III): Visible Region Detection, Red Fluorescence, and Human Breast Cancer Cell Imaging. RSC Adv. 2015, 5, 24194–24199. [Google Scholar] [CrossRef]
- Liu, G.; Shao, J. Selective Ratiometric Fluorescence Detection of Acetate Based on a Novel Schiff Base Derivative. J. Fluoresc. 2012, 22, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Ganjali, M.R.; Veismohammadi, B.; Faridbod, F.; Abkenard, S.D.; Salavati-Niasarie, M. Selective Recognition of Acetate Ion Based on Fluorescence Enhancement Chemosensor. Luminescence. 2012, 27, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, H.; Cai, Z.; Lin, H. Colorimetric Sensing of Biologically Important Acetate Ion Based on Indole Derivation. Mini-Rev. Org. Chem. 2011, 8, 25–30. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Wang, G.K.; Chen, J.H.; Zhang, L.M.; Liu, B.; Zhang, J.F.; Zhao, Q.H.; Zhou, Y. 1,8-Naphthalimide-based Visible Colorimetric Sensor for the Selective Sensing of Fluoride, Acetate, and Hydroxyl Anions. J. Fluorine Chem. 2014, 158, 53–59. [Google Scholar] [CrossRef]
- Wang, G.; Zheng, T.; Zhang, S.; Ye, J.; Ning, G. Fluorescence Chemosensor for Acetate Ion and Fluorine Ion based on 1,2,4-Triazolyl Substituted Pentaphenylpyridinium. J. Mol. Struct. 2021, 1230, 129918. [Google Scholar] [CrossRef]
- Goswami, S.; Das, A.K.; Aich, K.; Manna, A. Competitive Intra- and Inter-Molecular Proton Transfer in Hydroxynaphthyl Benzothiazole: Selective Ratiometric Sensing of Acetate. Tetrahedron Lett. 2013, 54, 4215–4220. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, X.; Wei, T.B.; Zhang, Y.M. TICT–ICT State Change Mechanism-based Acetate Fluorescent Sensor Act as an “Off–On–Off” Switch and Logic Gate. Sens. Actuators B. 2014, 190, 459–463. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noushija, M.K.; Shanmughan, A.; Mohan, B.; Shanmugaraju, S. Selective Recognition and Reversible “Turn-Off” Fluorescence Sensing of Acetate (CH3COO−) Anion at Ppb Level Using a Simple Quinizarin Fluorescent Dye. Chemistry 2022, 4, 1407-1416. https://doi.org/10.3390/chemistry4040092
Noushija MK, Shanmughan A, Mohan B, Shanmugaraju S. Selective Recognition and Reversible “Turn-Off” Fluorescence Sensing of Acetate (CH3COO−) Anion at Ppb Level Using a Simple Quinizarin Fluorescent Dye. Chemistry. 2022; 4(4):1407-1416. https://doi.org/10.3390/chemistry4040092
Chicago/Turabian StyleNoushija, Mannanthara Kunhumon, Ananthu Shanmughan, Binduja Mohan, and Sankarasekaran Shanmugaraju. 2022. "Selective Recognition and Reversible “Turn-Off” Fluorescence Sensing of Acetate (CH3COO−) Anion at Ppb Level Using a Simple Quinizarin Fluorescent Dye" Chemistry 4, no. 4: 1407-1416. https://doi.org/10.3390/chemistry4040092