Sorption-Assisted Ultrafiltration Hybrid Method for Treatment of the Radioactive Aqueous Solutions
Abstract
:1. Introduction
2. Experimental Details
2.1. Chemicals and Sorbent
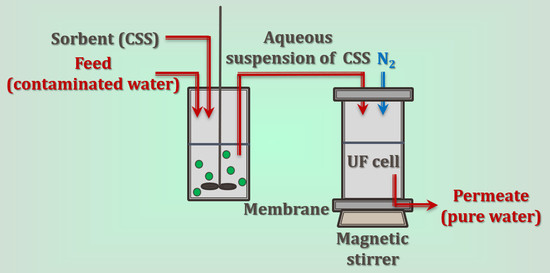
2.2. Laboratory Ultrafiltration Kit
2.3. Studies on the Water Purification
2.4. Methods Used in the Work and the Relevant Instrumentation
3. Results and Discussion
3.1. Purification of the Contaminated Water
3.1.1. Removal of Cationic Metal Species: Cs(I), Co(II) and Am(III)
3.1.2. Removal of Anionic Metal Species (TcO4−)
3.1.3. Treatment of the Simulated Liquid Radioactive Waste
3.2. Discussion of the Results in Relation to the Previous Outcomes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- INES. The International Nuclear and Radiological Event Scale User’s Manual, 2008th ed.; International Atomic Energy Agency: Vienna, Austria, 2013; Available online: https://www-pub.iaea.org/MTCD/Publications/PDF/INES2013web.pdf (accessed on 7 March 2022).
- Steinhauser, G.; Brandl, A.; Johnson, T.E. Comparison of the Chernobyl and Fukushima nuclear accidents: A review of the environmental impacts. Sci. Total Environ. 2014, 470–471, 800–817. [Google Scholar] [CrossRef] [PubMed]
- Environmental Consequences of the Chernobyl Accident and Their Remediation: Twenty Years of Experience; Report of the Chernobyl Forum Expert Group ‘Environment’; International Atomic Energy Agency: Vienna, Austria, 2006; p. 49. Available online: https://www-pub.iaea.org/MTCD/publications/PDF/Pub1239_web.pdf (accessed on 8 March 2022).
- Guidelines for Drinking-Water Quality, First Addendum to Third Edition; Volume 1: Recommendations; World Health Organization: Geneva, Switzerland, 2006; pp. 203–204. Available online: https://www.who.int/water_sanitation_health/dwq/gdwq0506.pdf (accessed on 7 March 2022).
- Rahman, R.O.A.; Ibrahim, H.A.; Hung, Y.-T. Liquid radioactive wastes treatment: A review. Water 2011, 3, 551–565. [Google Scholar] [CrossRef]
- Crini, G.; Morin-Crini, N.; Fatin-Rouge, N.; Déon, S.; Fievet, P. Metal removal from aqueous media by polymer-assisted ultrafiltration with chitosan. Arab. J. Chem. 2017, 10, S3826–S3839. [Google Scholar] [CrossRef]
- Maureira, A.; Rivas, B.L. Metal ions recovery with alginic acid coupled to ultrafiltration membrane. Eur. Polym. J. 2009, 45, 573–581. [Google Scholar] [CrossRef]
- Vanderberg, L.A.; Foreman, T.M.; Attrep, M., Jr.; Brainard, J.R.; Sauer, N. Treatment of heterogeneous mixed wastes: Enzyme degradation of cellulosic materials contaminated with hazardous organics and toxic and radioactive metals. Environ. Sci. Technol. 1999, 33, 1256–1263. [Google Scholar] [CrossRef]
- Rivas, B.L.; Urbano, B.F.; Sánchez, J. Water-Soluble and Insoluble Polymers, Nanoparticles, Nanocomposites and Hybrids with Ability to Remove Hazardous Inorganic Pollutants in Water. Front. Chem. 2018, 6, 320. [Google Scholar] [CrossRef] [PubMed]
- Hooper, E.W. The Combination of Finely Divided Inorganic Ion Exchangers and Ultrafiltration for the Treatment of Low- and Medium-Level Waste. In New Separation Chemistry Techniques for Radioactive Waste and Other Specific Applications; Cecille, S.L., Casarci, M., Pietrelli, L., Eds.; Springer: Amsterdan, The Netherlands, 1991; pp. 244–252. ISBN 1-85166-656-7. [Google Scholar]
- Chmielewski, A.G.; Harasimowicz, M.; Chudziak, A. Purification of liquid radioactive wastes by the ultrafiltration method connected with ion exchange. 2 National scientific conference on process engineering in environment protection. In Proceedings of the Conference Materials, Jachranka, Poland, 19–20 September 1994; pp. 227–231. Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/27/011/27011016.pdf?r=1 (accessed on 31 August 2022). (In Polish).
- Cojocaru, C.; Zakrzewska-Trznadel, G.; Jaworska, A. Removal of cobalt ions from aqueous solutions by polymer assisted ultrafiltration using experimental design approach. Part 1: Optimization of complexation conditions. J. Hazard. Mater. 2009, 169, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Dambies, L.; Jaworska, A.; Zakrzewska-Trznadel, G.; Sartowska, B. Comparison of acidic polymers for the removal of cobalt from water solutions by polymer assisted ultrafiltration. J. Hazard. Mater. 2010, 178, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Miśkiewicz, A.; Zakrzewska-Kołtuniewicz, G. Application of biosorbents in hybrid ultrafiltration/sorption processes to remove radionuclides from low-level radioactive waste. Desalination Water Treat. 2021, 242, 47–55. [Google Scholar] [CrossRef]
- Advances in Technologies for Treatment of Low and Intermediate Level Radioactive Liquid Wastes; Technical Reports Series No. 370; IAEA: Vienna, Austria, 1994.
- Cumming, I.W.; Williams, G.H.; Gutman, R.G.; Davison, C.G. Development of Combined Precipitation and Ultrafiltration Process and Its Application to the Treatment of Low Active Wastes; IAEA-SM-303-21P; IAEA: Vienna, Australia, 1992; p. 26. [Google Scholar]
- Hooper, E.W. Activity removal from aqueous waste streams by seeded ultrafiltration. In W: Use of Inorganic Sorbents for Treatment of Liquid Radioactive Waste and Backfill of Underground Repositories; IAEA-TECDOC-675; IAEA: Vienna, Austria, 1992; pp. 69–83. [Google Scholar]
- Hooper, E.W. Inorganic sorbents for removal of radioactivity from aqueous waste streams. In Waste Treatment and Immobilization Technologies Involving Inorganic Sorbents; IAEA-TECDOC-947; IAEA: Vienna, Australia, 1997; pp. 221–235. [Google Scholar]
- Waste Treatment and Immobilization Technologies Involving Inorganic Sorbents: Final Report of a Coordinated Research Programme 1992–1996; IAEA-TECDOC-947; International Atomic Energy Agency (IAEA): Vienna, Austria, 1997; pp. 221–236.
- Fuks, L.; Herdzik-Koniecko, I.; Maskalchuk, L.; Leontieva, T. Clay-salt slimes of the JSC “Belaruskali” as potential engineering barriers in the radioactive waste repositories: Sorption of Cs(I), Sr(II), Eu(III) and Am(III). Int. J. Environ. Sci. Technol. 2018, 15, 2047–2058. [Google Scholar] [CrossRef] [Green Version]
- Fuks, L.; Herdzik-Koniecko, I.; Maskalchuk, L.; Leontieva, T. Application of Industrial Wastes as an Engineering Barrier in Radioactive Waste Repositories: Sorption of Cs(I), Sr(II), Eu(III), and Am(III) Ions on the Clay-Salt Slimes of the Joint Stock Company “Belaruskali”; In the Institute of Nuclear Chemistry and Technology Annual Report 2016; Institute of Nuclear Chemistry and Technology: Warszawa, Poland, 2017; pp. 48–53. ISSN 1425-204X. [Google Scholar]
- Zhang, X.; Niu, L.; Li, F.; Yu, S.; Zhao, X.; Hu, H. Effect of gamma-ray irradiation at low doses on the performance of PES ultrafiltration membrane. Radiat. Phys. Chem. 2016, 127, 127–132. [Google Scholar] [CrossRef]
- Severa, J.; Bár, J. Handbook of Radioactive Contamination and Decontamination; Elsevier: Amsterdam, The Netherlands, 1991; Volume 47, ISBN 0-444-98757-6. [Google Scholar]
- van der Bruggen, B.; Vandecasteele, C.; van Gestel, T.; Doyen, W.; Leysen, R. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 2003, 22, 46–56. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2004; p. 8. ISBN 0-470-85445-6. [Google Scholar]
- Fane, A.G.; Chong, T.H.; Le-Clech, P. Fouling in Membrane Processes. In Membrane Operations. Innovative Separations and Transformations; Drioli, E., Giorno, L., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009. [Google Scholar]
- Chotkowski, M.; Czerwinski, A. Electrochemistry of Technetium; Springer: Cham, Switzerland, 2021; p. 5. ISBN 978-3-030-62863-5. [Google Scholar]
- Yoshihara, K.; Omori, T. (Eds.) Technetium and Rhenium Their Chemistry and Its Applications; Topics in Current Chemistry; Springer: Berlin, Germany, 1996; Volume 176, p. 18. ISBN 978-3-540-59469-7. [Google Scholar]
- Saha, G.B. Fundamentals of Nuclear Pharmacy, 5th ed.; Springer: New York, NY, USA, 2004; p. 73. ISBN 0-387-40360-4. [Google Scholar]
- Fuks, L.; Maskalchuk, L.; Herdzik-Koniecko, I.; Leontieva, T. Clay-salt slimes of the “Belaruskali”—Novel sorbents for management of liquid radioactive wastes and decontamination of environmental water streams. J. Radioanal. Nucl. Chem. 2019, 320, 87–100. [Google Scholar] [CrossRef]
- Obruchnikova, Y.; German, K.E.; Peretrukhin, V.F.; Logunov, M.V.; Mashkin, A.N. Behavior of Technetium in Nitric Acid Solutions, Containing Hydrazine Nitrate and Multi-Charges Metal Cations (Th and Zr). Available online: https://www.researchgate.net/publication/343106577_BEHAVIOR_OF_TECHNETIUM_IN_NITRIC_ACID_SOLUTIONS_CONTAINING_HYDRAZINE_NITRATE_AND_MULTI-CHARGES_METAL_CATIONS_Th_and_Zr (accessed on 14 August 2022).
- Annual Report: Activities of the President of National Agency for Nuclear Energy on the State of Nuclear Safety and Radiological Protection in Poland in 2021, Warsaw, 2022, p. 46. Available online: https://www.gov.pl/attachment/df08ef6c-7b67-4ce8-8912-b394481c46d5 (accessed on 14 August 2022). (In Polish)
- Lukasz, B.A.K. Management of Radioactive Waste in Poland 2016; Radioactive Waste Management Plant, Otwock-Swierk, Poland. Available online: https://conferences.iaea.org/event/106/contributions/1677/attachments/1166/1427/PAPER.pdf (accessed on 14 August 2022).
- Regulation of the Minister of Health of 7 December 2017 on the Quality of Water Intended for Human Consumption, Journal of Laws of 2017, Item 2294. Available online: https://www.infor.pl/akt-prawny/DZU.2017.240.0002294,rozporzadzenie-ministra-zdrowia-w-sprawie-jakosci-wody-przeznaczonej-do-spozycia-przez-ludzi.html?msclkid=ed02d263c5f911ec8e35ab579013c7d5 (accessed on 27 April 2022). (In Polish).
- Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-154995-0.
- Katsou, E.; Malamis, S.; Haralambous, K.J. Industrial wastewater pre-treatment for heavy metal reduction by employing a sorbent-assisted ultrafiltration system. Chemosphere 2011, 82, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Vakalis, S.; Moustakas, K.; Malamis, F.; Sotiropoulos, A.; Malamis, S. Assessing the removal of heavy metals in industrial wastewater by means of chemical exergy. Desalination Water Treat 2017, 91, 146–151. [Google Scholar] [CrossRef]
- Maskalchuk, L.N.; Milyutin, V.V.; Nekrasova, N.A.; Leontieva, T.G.; Baklay, A.A.; Belousov, P.E.; Krupskaya, V.V. Aluminosilicate sorbents based on clay-salt slimes from JSC “Belaruskali” for sorption of cesium and strontium radionuclides. Radiochemistry 2020, 62, 381–386. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Akar, T.; Tunali, S.; Cabuk, A.S. Study on the characterization of lead(II) biosorption by fungus aspergillus parasiticus. Appl. Biochem. Biotechnol. 2007, 136, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Undabeytia, T.; Nir, S.; Rytwo, C.; Serban, C.; Morillo, E.; Maqueda, C. Modeling adsorption-desorption processes of Cu on edge and planar sites of montmorillonit. Environ. Sci. Technol. 2002, 36, 2677–2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, G.B. Fundamentals of Nuclear Pharmacy, 7th ed.; Springer International Publishing: Cham, Switzerland, 2018; p. 111. ISBN 978-1-44195-859-4. [Google Scholar]
- Rapparini, R.; Tavoni, R.; Travaqlini, N. Fission and Corrosion Product Activities Studied in Italy (IWGFR--7); International Atomic Energy Agency (IAEA): Vienna, Austria, 1976. [Google Scholar]









| Description of the Experiment | Methods | Devices |
|---|---|---|
| Production of the saline aqueous solutions containing Tc-99m | Elution from the isotope generator; typical medical procedure | Mo-99/Tc-99m medical generator; GE Healthcare, (supplied by Biker, Warsaw, Poland) |
| Ultrafiltration (UF) | Sorbent-assisted ultrafiltration (UF) in the laboratory scale | The polyethersulfone (PES) ultrafiltration membrane (Merck Millipore) |
| The magnetically stirred membrane ultrafiltration cell AMICON 8400 (Merck Millipore) | ||
| Radiometric analyses of water | Well-type radiometric counting of gamma radiation | Perkin Elmer 2480 Wizard2© Automatic Gamma Counter (Waltham, MA, USA) |
| Checking purity of the radionuclides | Gamma radiation spectrometry | Gamma ray spectrometer with HPGe semiconductor detector (ORTEC, Canberra, Australia) with efficiency from 30 to 45% |
| Checking chemical composition of aqueous solutions | Ion chromatography | Ion chromatograph DIONEX ICS-5000 DC (DIONEX, Sunnyvale, CA, USA) |
| Cation */Anion | Raw Water | Permeate |
|---|---|---|
| [mg L−1] | ||
| Na+ | 1961.6 | 205.5 |
| K+ | 752.6 | 73.1 |
| Mg2+ | 121.1 | 5.8 |
| Ca2+ | 18.1 | 1.3 |
| Cl− | 219.5 | 193.4 |
| NO3− | 350.2 | 330.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuks, L.; Miśkiewicz, A.; Zakrzewska-Kołtuniewicz, G. Sorption-Assisted Ultrafiltration Hybrid Method for Treatment of the Radioactive Aqueous Solutions. Chemistry 2022, 4, 1076-1091. https://doi.org/10.3390/chemistry4030073
Fuks L, Miśkiewicz A, Zakrzewska-Kołtuniewicz G. Sorption-Assisted Ultrafiltration Hybrid Method for Treatment of the Radioactive Aqueous Solutions. Chemistry. 2022; 4(3):1076-1091. https://doi.org/10.3390/chemistry4030073
Chicago/Turabian StyleFuks, Leon, Agnieszka Miśkiewicz, and Grażyna Zakrzewska-Kołtuniewicz. 2022. "Sorption-Assisted Ultrafiltration Hybrid Method for Treatment of the Radioactive Aqueous Solutions" Chemistry 4, no. 3: 1076-1091. https://doi.org/10.3390/chemistry4030073