Using Highly Flexible SbSn@NC Nanofibers as Binderless Anodes for Sodium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Material Preparation
2.1.1. Preparation of SnO2 and Sb2O3 Nanomaterials
2.1.2. Preparation of SnSb Alloy–Carbon Nanofibers
2.2. Material Characterization
2.3. Electrochemical Measurements
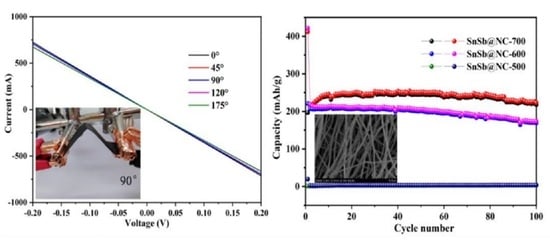
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, D.; Zhu, C.; Wu, M.; Wang, H.; Huang, J.; Tang, D.; Ma, J. Highly Oxidation-Resistant Electrolyte for 4.7 V Sodium Metal Batteries Enabled by Anion/Cation Solvation Engineering. Angew. Chem. Int. Ed. 2022, 61, e202214198. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Liu, H.; Zhao, X.S. Surface engineering of anode materials for improving sodium-ion storage performance. J. Mater. Chem. A 2022, 10, 3889–3904. [Google Scholar] [CrossRef]
- Lei, D.; He, Y.-B.; Huang, H.; Yuan, Y.; Zhong, G.; Zhao, Q.; Hao, X.; Zhang, D.; Lai, C.; Zhang, S.; et al. Cross-linked beta alumina nanowires with compact gel polymer electrolyte coating for ultra-stable sodium metal battery. Nat. Commun. 2019, 10, 4244. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Wei, Z.; Wang, C.; Ma, J. Vacancy-induced sodium-ion storage in N-doped carbon Nanofiber@MoS2 nanosheet arrays. Electrochim. Acta 2018, 285, 301–308. [Google Scholar] [CrossRef]
- Li, S.; He, W.; Liu, B.; Cui, J.; Wang, X.; Peng, D.-L.; Liu, B.; Qu, B. One-step construction of three-dimensional nickel sulfide-embedded carbon matrix for sodium-ion batteries and hybrid capacitors. Energy Storage Mater. 2019, 25, 636–643. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, D.; Liu, Y.; Wang, J.; Li, Z.; Li, X.; Han, G.; Wei, Q.; Qu, B. Sodium Stoichiometry Tuning of the Biphasic-NaxMnO2 Cathode for High-Performance Sodium-Ion Batteries. Small 2023, 61, e2301141. [Google Scholar] [CrossRef]
- Liang, J.; Fan, K.; Wei, Z.; Gao, X.; Song, W.; Ma, J. Porous NaTi2(PO4)3@C nanocubes as improved anode for sodium-ion batteries. Mater. Res. Bull. 2018, 99, 343–348. [Google Scholar] [CrossRef]
- Qi, S.; Wu, D.; Dong, Y.; Liao, J.; Foster, C.W.; O’Dwyer, C.; Feng, Y.; Liu, C.; Ma, J. Cobalt-based electrode materials for sodium-ion batteries. Chem. Eng. J. 2019, 370, 185–207. [Google Scholar] [CrossRef]
- Wang, L.; Yang, C.; Dou, S.; Wang, S.; Zhang, J.; Gao, X.; Ma, J.; Yu, Y. Nitrogen-doped hierarchically porous carbon networks: Synthesis and applications in lithium-ion battery, sodium-ion battery and zinc-air battery. Electrochim. Acta 2016, 219, 592–603. [Google Scholar] [CrossRef]
- Wang, L.; Wei, Z.; Mao, M.; Wang, H.; Li, Y.; Ma, J. Metal oxide/graphene composite anode materials for sodium-ion batteries. Energy Storage Mater. 2019, 16, 434–454. [Google Scholar] [CrossRef]
- Chen, C.; Yang, Y.; Tang, X.; Qiu, R.; Wang, S.; Cao, G.; Zhang, M. Graphene-Encapsulated FeS2 in Carbon Fibers as High Reversible Anodes for Na+/K+ Batteries in a Wide Temperature Range. Small 2019, 15, e1804740. [Google Scholar] [CrossRef]
- Liang, J.-M.; Zhang, L.-J.; Xili, D.-G.; Kang, J. Research progress on tin-based anode materials for sodium ion batteries. Rare Met. 2020, 39, 1005–1018. [Google Scholar] [CrossRef]
- Luo, W.; Gaumet, J.-J.; Mai, L.-Q. Antimony-based intermetallic compounds for lithium-ion and sodium-ion batteries: Synthesis, construction and application. Rare Met. 2017, 36, 321–338. [Google Scholar] [CrossRef]
- Jing, W.T.; Yang, C.C.; Jiang, Q. Recent progress on metallic Sn- and Sb-based anodes for sodium-ion batteries. J. Mater. Chem. A 2020, 8, 2913–2933. [Google Scholar] [CrossRef]
- Sarkar, S.; Peter, S.C. An overview on Sb-based intermetallics and alloys for sodium-ion batteries: Trends, challenges and future prospects from material synthesis to battery performance. J. Mater. Chem. A 2021, 9, 5164–5196. [Google Scholar] [CrossRef]
- Li, L.; Seng, K.H.; Li, D.; Xia, Y.; Liu, H.K.; Guo, Z. SnSb@carbon nanocable anchored on graphene sheets for sodium ion batteries. Nano Res. 2014, 7, 1466–1476. [Google Scholar] [CrossRef]
- Walter, M.; Doswald, S.; Kovalenko, M.V. Inexpensive colloidal SnSb nanoalloys as efficient anode materials for lithium- and sodium-ion batteries. J. Mater. Chem. A 2016, 4, 7053–7059. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Dirican, M.; Chen, C.; Zhu, J.; Zhu, P.; Yan, C.; Li, Y.; Dong, X.; Guo, J.; Zhang, X. Reduced graphene oxide-incorporated SnSb@CNF composites as anodes for high-performance sodium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 9696–9703. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Zhao, Z.; Hu, Z.; Liu, X.; Yu, G. Flexible sodium-ion based energy storage devices: Recent progress and challenges. Energy Storage Mater. 2019, 26, 83–104. [Google Scholar] [CrossRef]
- Mishra, K.; Yadav, N.; Hashmi, S.A. Recent progress in electrode and electrolyte materials for flexible sodium-ion batteries. J. Mater. Chem. A 2020, 8, 22507–22543. [Google Scholar] [CrossRef]
- Tang, X.; Yan, F.; Wei, Y.; Zhang, M.; Wang, T.; Zhang, T. Encapsulating SnxSb Nanoparticles in Multichannel Graphene-Carbon Fibers As Flexible Anodes to Store Lithium Ions with High Capacities. ACS Appl. Mater. Interfaces 2015, 7, 21890–21897. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Long, Y.-Z.; Chen, Z.-J.; Liu, S.-L.; Zhang, H.-D.; Zhang, J.-C.; Han, W.-P. Recent advances in flexible and stretchable electronic devices via electrospinning. J. Mater. Chem. C 2013, 2, 1209–1219. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Ni, J.; Li, L. Electrospinning for flexible sodium-ion batteries. Energy Storage Mater. 2022, 45, 704–719. [Google Scholar] [CrossRef]
- Xiong, Q.; He, H.; Zhang, M. Design of Flexible Films Based on Kinked Carbon Nanofibers for High Rate and Stable Potassium-Ion Storage. Nano-Micro Lett. 2022, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, Y.; Hu, Y.; Ruan, H.; Bai, J.; Li, S.; Liu, Y.; Guo, S. Flexible Sb/Sb2O3-C nanofibers as binder-free anodes for high-performance and stable sodium-ion batteries. J. Alloys Compd. 2021, 890, 161913. [Google Scholar] [CrossRef]
- Sadan, M.K.; Kim, H.; Kim, C.; Cho, G.-B.; Cho, K.-K.; Ahn, J.-H.; Ahn, H.-J. Ultra-long cycle life of flexible Sn anode using DME electrolyte. J. Alloys Compd. 2021, 871, 159549. [Google Scholar] [CrossRef]
- Zhang, Q.; Zeng, Y.; Ling, C.; Wang, L.; Wang, Z.; Fan, T.; Wang, H.; Xiao, J.; Li, X.; Qu, B. Boosting Fast Sodium Ion Storage by Synergistic Effect of Heterointerface Engineering and Nitrogen Doping Porous Carbon Nanofibers. Small 2022, 18, 2107514. [Google Scholar] [CrossRef]
- He, H.; Lian, J.; Chen, C.; Xiong, Q.; Li, C.C.; Zhang, M. Enabling Multi-Chemisorption Sites on Carbon Nanofibers Cathodes by an In-situ Exfoliation Strategy for High-Performance Zn–Ion Hybrid Capacitors. Nano-Micro Lett. 2022, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wu, D.; Zhu, J.; Xie, J.; Wu, J.; Liang, J. One-dimensional ZnSe@N-doped carbon nanofibers with simple electrospinning route for superior Na/K-ion storage. Chin. Chem. Lett. 2023, 34, 107416. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Wu, Q.; Zhang, Q.; Chen, H.; Jing, H.; Wang, J.; Mi, S.-B.; Rogach, A.L.; Niu, C. Encapsulating Silica/Antimony into Porous Electrospun Carbon Nanofibers with Robust Structure Stability for High-Efficiency Lithium Storage. ACS Nano 2018, 12, 3406–3416. [Google Scholar] [CrossRef]
- Joshi, B.; Samuel, E.; Kim, Y.-I.; Yarin, A.L.; Swihart, M.T.; Yoon, S.S. Progress and potential of electrospinning-derived substrate-free and binder-free lithium-ion battery electrodes. Chem. Eng. J. 2021, 430, 132876. [Google Scholar] [CrossRef]
- Liu, B.; Lei, D.; Wang, J.; Zhang, Q.; Zhang, Y.; He, W.; Zheng, H.; Sa, B.; Xie, Q.; Peng, D.-L.; et al. 3D uniform nitrogen-doped carbon skeleton for ultra-stable sodium metal anode. Nano Res. 2020, 13, 2136–2142. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, J.; Wang, C.; Ma, J.; Wallace, G.G. Tunable and Efficient Tin Modified Nitrogen-Doped Carbon Nanofibers for Electrochemical Reduction of Aqueous Carbon Dioxide. Adv. Energy Mater. 2018, 8, 1702524. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Yuan, C.; Li, H.; Fan, K.; Wei, Z.; Sun, H.; Ma, J. Growth of SnO2 Nanoflowers on N-doped Carbon Nanofibers as Anode for Li- and Na-ion Batteries. Nano-Micro Lett. 2017, 10, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, J.; Zhu, R.; Zhang, S.; Yang, L.; Chen, X.; Wang, H.; Liu, X. Bean Pod-Like SbSn/N-Doped Carbon Fibers toward a Binder Free, Free-Standing, and High-Performance Anode for Sodium-Ion Batteries. Small 2022, 18, 2107869. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, L.; Zhao, X.; Wu, L.; Wang, K.; Si, H.; Gu, J.; Sun, C.; Shi, Y.; Zhang, Y. Construction of Sn–P–graphene microstructure with Sn–C and P–C co-bonding as anodes for lithium-ion batteries. Chem. Commun. 2020, 56, 10572–10575. [Google Scholar] [CrossRef]
- Dai, C.; Zhou, Z.; Zhou, X.; Zhang, Y. Removal of Sb(III) and Sb(V) from Aqueous Solutions Using nZVI. Water Air Soil Pollut. 2013, 225, 1799. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Xu, X.; Yan, J.; Ma, G.; Lei, Z. Anchoring Sb6O13 nanocrystals on graphene sheets for enhanced lithium storage. ACS Appl. Mater. Interface 2016, 8, 35398–35406. [Google Scholar] [CrossRef]
- Javed, R.; Khan, M.A.; Ye, D.; Zhao, Y.; Shah, L.A.; Zhang, J.; Zhao, H. Boosting Oxygen Reduction Catalysis Through Electronic Reconfiguration of Fe–N–C Induced by P Doping. Electrocatalysis 2021, 12, 747–758. [Google Scholar] [CrossRef]
- Cheng, D.; Wei, A.; Ye, L.; Xu, G.; Tan, L.; Lu, B.; Chen, Y. Interfacial Bonding of SnSb Alloys with Graphene toward Ultrafast and Cycle-Stable Na-Ion Battery Anodes. ACS Sustain. Chem. Eng. 2022, 10, 12177–12187. [Google Scholar] [CrossRef]
- Yang, L.; Yang, B.; Chen, X.; Wang, H.; Dang, J.; Liu, X. Bimetallic alloy SbSn nanodots filled in electrospun N-doped carbon fibers for high performance Na-ion battery anode. Electrochim. Acta 2021, 389, 138246. [Google Scholar] [CrossRef]
- Song, Z.; Wang, G.; Chen, Y.; Lu, Y.; Wen, Z. In situ three-dimensional cross-linked carbon nanotube-interspersed SnSb@CNF as freestanding anode for long-term cycling sodium-ion batteries. Chem. Eng. J. 2023, 463, 142289. [Google Scholar] [CrossRef]
- Jia, H.; Dirican, M.; Zhu, J.; Chen, C.; Yan, C.; Zhu, P.; Li, Y.; Guo, J.; Caydamli, Y.; Zhang, X. High-performance SnSb@rGO@CMF composites as anode material for sodium-ion batteries through high-speed centrifugal spinning. J. Alloys Compd. 2018, 752, 296–302. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Fang, G.; Niu, X.; Zhang, Z.; Wang, Y.; Liao, L.; Zheng, X.; Huang, D.; Wei, Y. Using Highly Flexible SbSn@NC Nanofibers as Binderless Anodes for Sodium-Ion Batteries. Surfaces 2023, 6, 239-248. https://doi.org/10.3390/surfaces6030016
Liang J, Fang G, Niu X, Zhang Z, Wang Y, Liao L, Zheng X, Huang D, Wei Y. Using Highly Flexible SbSn@NC Nanofibers as Binderless Anodes for Sodium-Ion Batteries. Surfaces. 2023; 6(3):239-248. https://doi.org/10.3390/surfaces6030016
Chicago/Turabian StyleLiang, Jiaojiao, Gengkun Fang, Xinmiao Niu, Zhihao Zhang, Yufei Wang, Lingyuan Liao, Xiaoming Zheng, Di Huang, and Yuehua Wei. 2023. "Using Highly Flexible SbSn@NC Nanofibers as Binderless Anodes for Sodium-Ion Batteries" Surfaces 6, no. 3: 239-248. https://doi.org/10.3390/surfaces6030016