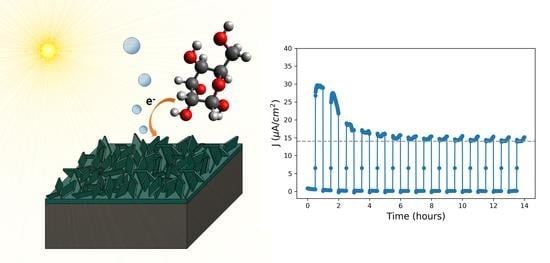
Co3O4 Nanopetals on Si as Photoanodes for the Oxidation of Organics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Structural and Morphological Characterizations
2.3. Electrochemical Measurements
3. Results and Discussion
3.1. Structure and Activity
3.2. Mechanistic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| UHV | Ultra High Vacuum |
| FE-SEM | Field Emission- Scanning Electron Microscopy |
| GIXRD | Grazing Incidence X-Ray Diffraction |
| VB | Valence Band |
| CB | Conduction Band |
| EIS | Electrochemical Impedance Spectroscopy |
| PEC | PhotoElectroChemical |
| COD | Chemical Oxygen Demand |
| LSV | Linear Sweep Voltammetry |
| XPS | X-Ray Photoelectron Spectroscopy |
References
- Cesar, I.; Sivula, K.; Kay, A.; Zboril, R.; Grätzel, M. Influence of Feature Size, Film Thickness, and Silicon Doping on the Performance of Nanostructured Hematite Photoanodes for Solar Water Splitting. J. Phys. Chem. C 2009, 113, 772–782. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, B.; Zheng, Q.; Li, J.; Bai, J.; Liu, Y.; Cai, W. Photoelectrocatalytic COD determination method using highly ordered TiO2 nanotube array. Water Res. 2009, 43, 1986–1992. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Zhou, M.; Martínez-Huitle, C.A. Heterogeneous electro-Fenton and photoelectro-Fenton processes: A critical review of fundamental principles and application for water/wastewater treatment. Appl. Catal. B Environ. 2018, 235, 103–129. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Brillas, E. Applied photoelectrocatalysis on the degradation of organic pollutants in wastewaters. J. Photochem. Photobiol. C Photochem. Rev. 2017, 31, 1–35. [Google Scholar]
- Shi, Y.; Han, T.; Gimbert-Suriñach, C.; Song, X.; Lanza, M.; Llobet, A. Substitution of native silicon oxide by titanium in Ni-coated silicon photoanodes for water splitting solar cells. J. Mater. Chem. A 2017, 5, 1996–2003. [Google Scholar] [CrossRef]
- Han, T.; Shi, Y.; Song, X.; Mio, A.; Valenti, L.; Hui, F.; Privitera, S.; Lombardo, S.; Lanza, M. Ageing mechanisms of highly active and stable nickel-coated silicon photoanodes for water splitting. J. Mater. Chem. A 2016, 4, 8053–8060. [Google Scholar] [CrossRef]
- Li, L.; Duan, L.; Xu, Y.; Gorlov, M.; Hagfeldt, A.; Sun, L. A photoelectrochemical device for visible light driven water splitting by a molecular ruthenium catalyst assembled on dye-sensitized nanostructured TiO2. Chem. Commun. 2010, 46, 7307–7309. [Google Scholar] [CrossRef]
- Kenney, M.J.; Gong, M.; Li, Y.; Wu, J.Z.; Feng, J.; Lanza, M.; Dai, H. High-Performance Silicon Photoanodes Passivated with Ultrathin Nickel Films for Water Oxidation. Science 2013, 342, 836–840. [Google Scholar] [CrossRef]
- Sun, K.; McDowell, M.T.; Nielander, A.C.; Hu, S.; Shaner, M.R.; Yang, F.; Brunschwig, B.S.; Lewis, N.S. Stable Solar-Driven Water Oxidation to O2(g) by Ni-Oxide-Coated Silicon Photoanodes. J. Phys. Chem. Lett. 2015, 6, 592–598. [Google Scholar] [CrossRef]
- Chen, Y.W.; Prange, J.D.; Duehnen, S.; Park, Y.; Gunji, M.; Chidsey, C.E.D.; McIntyre, P.C. Atomic layer-deposited tunnel oxide stabilizes silicon photoanodes for water oxidation. Nat. Mater. 2011, 10, 539–544. [Google Scholar] [CrossRef]
- Du, J.; Chen, Z.; Ye, S.; Wiley, B.J.; Meyer, T.J. Copper as a Robust and Transparent Electrocatalyst for Water Oxidation. Angew. Chem. Int. Ed. 2015, 54, 2073–2078. [Google Scholar] [CrossRef]
- Yu, F.; Li, F.; Zhang, B.; Li, H.; Sun, L. Efficient Electrocatalytic Water Oxidation by a Copper Oxide Thin Film in Borate Buffer. ACS Catal. 2015, 5, 627–630. [Google Scholar] [CrossRef]
- Strandwitz, N.C.; Comstock, D.J.; Grimm, R.L.; Nichols-Nielander, A.C.; Elam, J.; Lewis, N.S. Photoelectrochemical Behavior of n-type Si(100) Electrodes Coated with Thin Films of Manganese Oxide Grown by Atomic Layer Deposition. J. Phys. Chem. C 2013, 117, 4931–4936. [Google Scholar] [CrossRef] [Green Version]
- Artero, V.; Chavarot-Kerlidou, M.; Fontecave, M. Splitting Water with Cobalt. Angew. Chem. Int. Ed. 2011, 50, 7238–7266. [Google Scholar] [CrossRef]
- Kosmala, T.; Calvillo, L.; Agnoli, S.; Granozzi, G. Enhancing the Oxygen Electroreduction Activity through Electron Tunnelling: CoOx Ultrathin Films on Pd(100). ACS Catal. 2018, 8, 2343–2352. [Google Scholar] [CrossRef]
- Bae, D.; Mei, B.; Frydendal, R.; Pedersen, T.; Seger, B.; Hansen, O.; Vesborg, P.C.K.; Chorkendorff, I. Back-Illuminated Si-Based Photoanode with Nickel Cobalt Oxide Catalytic Protection Layer. ChemElectroChem 2016, 3, 1546–1552. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Walczak, K.; Anzenberg, E.; Toma, F.M.; Yuan, G.; Beeman, J.; Schwartzberg, A.; Lin, Y.; Hettick, M.; Javey, A.; et al. Efficient and Sustained Photoelectrochemical Water Oxidation by Cobalt Oxide/Silicon Photoanodes with Nanotextured Interfaces. J. Am. Chem. Soc. 2014, 136, 6191–6194. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, V.; Kim, H.; Park, J.; Yang, B. Cobalt oxide nanoparticles on TiO2 nanorod/FTO as a photoanode with enhanced visible light sensitization. RSC Adv. 2016, 6, 9789–9795. [Google Scholar] [CrossRef]
- Maeda, K.; Ishimaki, K.; Tokunaga, Y.; Lu, D.; Eguchi, M. Modification of Wide-Band-Gap Oxide Semiconductors with Cobalt Hydroxide Nanoclusters for Visible-Light Water Oxidation. Angew. Chem. Int. Ed. 2016, 55, 8309–8313. [Google Scholar] [CrossRef]
- Maeda, K.; Ishimaki, K.; Okazaki, M.; Kanazawa, T.; Lu, D.; Nozawa, S.; Kato, H.; Kakihana, M. Cobalt Oxide Nanoclusters on Rutile Titania as Bifunctional Units for Water Oxidation Catalysis and Visible Light Absorption: Understanding the Structure—Activity Relationship. ACS Appl. Mater. Interfaces 2017, 9, 6114–6122. [Google Scholar] [CrossRef]
- Wang, C.; Wu, J.; Wang, P.; Ao, Y.; Hou, J.; Qian, J. Photoelectrocatalytic determination of chemical oxygen demand under visible light using Cu2O-loaded TiO2 nanotube arrays electrode. Sens. Actuators B Chem. 2013, 181, 1–8. [Google Scholar]
- Zhang, Z.; Chang, X.; Chen, A. Determination of chemical oxygen demand based on photoelectrocatalysis of nanoporous TiO2 electrodes. Sens. Actuators B Chem. 2016, 223, 664–670. [Google Scholar]
- Hejzlar, J.; Kopáček, J. Determination of low chemical oxygen demand values in water by the dichromate semi-micro method. Analyst 1990, 115, 1463–1467. [Google Scholar] [CrossRef]
- Raider, S.; Flitsch, R.; Palmer, M. Oxide Growth on Etched Silicon in Air at Room Temperature. J. Electrochem. Soc. 1975, 122, 413–418. [Google Scholar] [CrossRef]
- Lutterotti, L.; Chateigner, D.; Ferrari, S.; Ricote, J. Texture, Residual Stress and Structural Analysis of Thin Films Using a Combined X-Ray Analysis. Thin Solid Films 2004, 450, 34–41. [Google Scholar] [CrossRef]
- Yu, T.; Zhu, Y.; Xu, X.; Shen, Z.; Chen, P.; Lim, C.T.; Thong, J.L.; Sow, C.H. Controlled Growth and Field-Emission Properties of Cobalt Oxide Nanowalls. Adv. Mater. 2005, 17, 1595–1599. [Google Scholar] [CrossRef]
- Xie, X.; Chung, H.; Sow, C.; Wee, A. Oxide growth and its dielectrical properties on alkylsilated native-SiO2/Si surface. Chem. Phys. Lett. 2004, 388, 446–451. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543. [Google Scholar] [CrossRef]
- Grządziel, L.; Krzywiecki, M.; Peisert, H.; Chassé, T.; Szuber, J. Photoemission study of the Si(111)-native SiO2/copper phthalocyanine (CuPc) ultra-thin film interface. Org. Electron. 2012, 13, 1873–1880. [Google Scholar]
- Qiao, L.; Xiao, H.Y.; Meyer, H.M.; Sun, J.N.; Rouleau, C.M.; Puretzky, A.A.; Geohegan, D.B.; Ivanov, I.N.; Yoon, M.; Weber, W.J.; Biegalski, M.D. Nature of the band gap and origin of the electro-/photo-activity of Co3O4. J. Mater. Chem. C 2013, 1, 4628–4633. [Google Scholar] [CrossRef]
- Guo, W.; Chemelewski, W.D.; Mabayoje, O.; Xiao, P.; Zhang, Y.; Mullins, C.B. Synthesis and Characterization of CuV2O6 and Cu2V2O7: Two Photoanode Candidates for Photoelectrochemical Water Oxidation. J. Phys. Chem. C 2015, 119, 27220–27227. [Google Scholar] [CrossRef]
- Hsu, Y.K.; Yu, C.H.; Chen, Y.C.; Lin, Y.G. Synthesis of novel Cu2O micro/nanostructural photocathode for solar water splitting. Electrochim. Acta 2013, 105, 62–68. [Google Scholar] [CrossRef]
- Cardon, F.; Gomes, W.P. On the determination of the flat-band potential of a semiconductor in contact with a metal or an electrolyte from the Mott-Schottky plot. J. Phys. D Appl. Phys. 1978, 11, L63. [Google Scholar]
- Muñoz, A. Semiconducting properties of self-organized TiO2 nanotubes. Electrochim. Acta 2007, 52, 4167–4176. [Google Scholar] [CrossRef]
- Dunn, H.K.; Feckl, J.M.; Müller, A.; Fattakhova-Rohlfing, D.; Morehead, S.G.; Roos, J.; Peter, L.M.; Scheu, C.; Bein, T. Tin doping speeds up hole transfer during light-driven water oxidation at hematite photoanodes. Phys. Chem. Chem. Phys. 2014, 16, 24610–24620. [Google Scholar] [CrossRef] [Green Version]
- Berger, T.; Monllor-Satoca, D.; Jankulovska, M.; Lana-Villarreal, T.; Gómez, R. The Electrochemistry of Nanostructured Titanium Dioxide Electrodes. ChemPhysChem 2012, 13, 2824–2875. [Google Scholar] [CrossRef]
- Chuang, T.; Brundle, C.; Rice, D. Interpretation of the X-ray photoemission spectra of cobalt oxides and cobalt oxide surfaces. Surf. Sci. 1976, 59, 413–429. [Google Scholar] [CrossRef]
- Dhara, K.; Mahapatra, D.R. Electrochemical nonenzymatic sensing of glucose using advanced nanomaterials. Microchim. Acta 2017, 185, 49. [Google Scholar] [CrossRef]
- Zonno, I.; Martinez-Otero, A.; Hebig, J.C.; Kirchartz, T. Understanding Mott-Schottky Measurements under Illumination in Organic Bulk Heterojunction Solar Cells. Phys. Rev. Appl. 2017, 7, 034018. [Google Scholar] [CrossRef]
- Zarean, M.; Keikha, M.; Poursafa, P.; Khalighinejad, P.; Amin, M.; Kelishadi, R. A systematic review on the adverse health effects of di-2-ethylhexyl phthalate. Environ. Sci. Pollut. Res. 2016, 23, 24642–24693. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Fan, S.; Zhao, M.; Ding, L.; Liang, J.; Chen, J.; Li, Y.; Chen, S. Synthesis of 3D hierarchical porous Co3O4 film by eggshell membrane for non-enzymatic glucose detection. J. Electroanal. Chem. 2016, 775, 52–57. [Google Scholar] [CrossRef]
- Li, M.; Han, C.; Zhang, Y.; Bo, X.; Guo, L. Facile synthesis of ultrafine Co3O4 nanocrystals embedded carbon matrices with specific skeletal structures as efficient non-enzymatic glucose sensors. Anal. Chim. Acta 2015, 861, 25–35. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Su, L.; Bellagamba, M.; Zhang, H.; Lei, Y. Electrospun Co3O4 nanofibers for sensitive and selective glucose detection. Biosens. Bioelectron. 2010, 26, 542–548. [Google Scholar] [CrossRef]
- George, G.; Anandhan, S. A comparative study on the physico–chemical properties of sol–gel electrospun cobalt oxide nanofibres from two different polymeric binders. RSC Adv. 2015, 5, 81429–81437. [Google Scholar] [CrossRef]
- Lopes, T.; Andrade, L.; Ribeiro, H.A.; Mendes, A. Characterization of photoelectrochemical cells for water splitting by electrochemical impedance spectroscopy. Int. J. Hydrog. Energy 2010, 35, 11601–11608. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Lu, Z.; Sun, X.; Jiang, L.; Duan, X. Superwetting Electrodes for Gas-Involving Electrocatalysis. Acc. Chem. Res. 2018, 51, 1590–1598. [Google Scholar] [CrossRef]
- Wang, S.; He, T.; Yun, J.H.; Hu, Y.; Xiao, M.; Du, A.; Wang, L. New Iron-Cobalt Oxide Catalysts Promoting BiVO4 Films for Photoelectrochemical Water Splitting. Adv Funct. Mater. 2018, 28, 1802685. [Google Scholar] [CrossRef]





| Sample Name | Treatment Conditions |
|---|---|
| PETAL | 4 h at 300 °C |
| HYBRID | 4 h at 300 °C + 1 h at 450 °C |
| NOPETAL | 1 h at 450 °C |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girardi, L.; Bardini, L.; Michieli, N.; Kalinic, B.; Maurizio, C.; Rizzi, G.A.; Mattei, G. Co3O4 Nanopetals on Si as Photoanodes for the Oxidation of Organics. Surfaces 2019, 2, 41-53. https://doi.org/10.3390/surfaces2010004
Girardi L, Bardini L, Michieli N, Kalinic B, Maurizio C, Rizzi GA, Mattei G. Co3O4 Nanopetals on Si as Photoanodes for the Oxidation of Organics. Surfaces. 2019; 2(1):41-53. https://doi.org/10.3390/surfaces2010004
Chicago/Turabian StyleGirardi, Leonardo, Luca Bardini, Niccolò Michieli, Boris Kalinic, Chiara Maurizio, Gian Andrea Rizzi, and Giovanni Mattei. 2019. "Co3O4 Nanopetals on Si as Photoanodes for the Oxidation of Organics" Surfaces 2, no. 1: 41-53. https://doi.org/10.3390/surfaces2010004