Invasive Pulmonary Aspergillosis in Coronavirus Disease 2019 Patients Lights and Shadows in the Current Landscape
Abstract
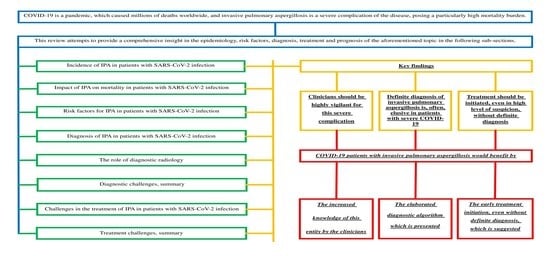
:Highlights
- A definite diagnosis of invasive pulmonary aspergillosis is elusive in patients with severe COVID-19.
- Experimental and clinical data indicate that delayed initiation of antifungal therapy could be detrimental to IA.
- The persistence of a respiratory co-infection in SARS-CoV-2 patients despite the administration of broad-spectrum antibiotics should lead to the pursuit of the confirmation or exclusion of IPA, especially in those patients who present risk factors for invasive pulmonary aspergillosis.
- Early treatment should be initiated, even in the absence of a definite diagnosis, when clinical suspicion is high.
Abstract
1. Introduction
2. Incidence, Risk Factors and Outcome of IPA in Patients with SARS-CoV-2 Infection
2.1. Incidence
- IPA frequently manifests with nonspecific symptoms and is not routinely suspected;
- Respiratory deterioration is considered to be caused by bacterial co-infection rather than fungal infection;
- Diagnosis of IA, frequently, requires invasive tissue specimens collection;
- Histopathologic identification is challenging;
- Cross-reaction of fungal antibody tests may exist;
- Lack of routine surveillance for IA is common.
2.2. Impact of IPA on Mortality in Patients with SARS-CoV-2 Infection
2.3. Risk Factors for IPA in Patients with SARS-CoV-2 Infection
3. Diagnosis of IPA in Patients with SARS-CoV-2 Infection
3.1. Diagnostic Criteria
3.2. The Role of Diagnostic Radiology
3.3. Diagnostic Challenges, Summary
- In light of the current difficulties and uncertainties relating to the diagnosis and the risks associated with IA in COVID-19 patients, clinicians should maintain a high level of suspicion for this infection, especially in ICU patients;
- The persistence of a respiratory co-infection in SARS-CoV-2 patients despite the administration of broad-spectrum antibiotics should lead to the pursuit of the confirmation or exclusion of IPA with culture- and non-culture-based methods, especially in those patients who present risk factors for IPA;
- Bronchoscopy has a limited role in these patients and should only be considered when diagnosis confirmation would significantly change clinical management;
- Conventional microscopic examination and qualitative culture of respiratory tract samples have quite low sensitivity and specificity;
- Confirmation test with blood biomarkers (serum GM or beta-D-glucan), blood PCR, or BAL GM or PCR, if possible, could be performed in cases of high clinical suspicion;
- The use of CT scans for diagnostic purposes is controversial due to practical concerns and the complex character of lesions presented in SARS-CoV-2 patients;
- Implementation of immuno-chromatographic LFD for the POC diagnosis of IPA could be helpful.
4. Challenges in the Treatment of IPA in Patients with SARS-CoV-2 Infection
Treatment Challenges, Summary
- Key objective is to improve survival, by avoiding misdiagnosis and by initiating early, targeted, and specific antifungal treatment. Any patient at risk should be considered by the responsible clinician as having IA and should receive antifungal therapy;
- There are possible drug–drug interactions between antifungal agents and agents used for specific treatment of coronavirus infection (tocilizumab-IL-6 receptor blocker-anakinra);
- The antifungal drug arsenal is very limited with high toxicity and severe side effects;
- Prolonged exposure to novel echinocandins (e.g., anidulafungin, micafungin), or triazoles (e.g., voriconazole, isavuconazole, and posaconazole) may result in the development of new resistance patterns leading to treatment failures;
- Lack of necessary equipment for microbiological examination, failure of early detection of fungal growth in infected tissue, incorrect technique of specimen sampling and clinicians’ failure to identify the precise fungi lead to high mortality rates;
- The optimal duration of antifungal therapy for CAPA is still under debate;
- Over-suppression of the immune system caused by the disease or the use of specific trial treatment (anakinra-recombinant IL-1Ra- or Janus kinase (JAK) inhibitors), might favor the rise of potential opportunistic fungal infections.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Abbreviation | Expansion |
|---|---|
| ARDS AspICU | Acute Respiratory Distress Syndrome Clinical Algorithm to Diagnose Invasive Pulmonary Aspergillosis in Critically Ill Patients (by Blot et.al., ref. [53]) |
| BAL | Bronchoalveolar Lavage |
| BDG | b-D-glucan |
| bmGT | bis(methylthio)gliotoxin |
| CAPA | COVID-19-Associated Pulmonary Aspergillosis |
| CDC | Centers for Disease Control and Prevention |
| COP | Cryptogenic organizing pneumonitis |
| COPD | Chronic Obstructive Pulmonary Disease |
| COVID-19 | Coronavirus Disease 2019 |
| CSF | Cerebrospinal Fluid |
| CT | Computed Tomography |
| CYP | Cytochrome P |
| ECMM | European Confederation of Medical Mycology |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EORTC | European Organization for Research and Treatment of Cancer |
| ERS | European Respiratory Society |
| ESCMID | European Society for Clinical Microbiology and Infectious Diseases |
| GAFFI | Global Action Fund for Fungal Infections |
| GM | Galactomannan |
| GM-EIA | Galactomannan Enzyme Immunoassay |
| GT | Gliotoxin |
| HIV | Human Immunodeficiency Virus |
| IA | Invasive Aspergillosis |
| IAPA | Influenza-Associated Pulmonary Aspergillosis |
| IDSA | Infectious Diseases Society of America |
| IFIs | Invasive Fungal Infections |
| IPA | Invasive Pulmonary Aspergillosis |
| JAK | Janus Kinase |
| LFD | Lateral Flow Device |
| Mab | Monoclonal Antibody |
| MSGERC | Mycoses Study Group Education and Research Consortium |
| OR | Odds Ratio |
| PCR | Polymerase Chain Reaction |
| POC | Point-Of-Care |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
References
- Morrell, M.; Fraser, V.J.; Kollef, M.H. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: A potential risk factor for hospital mortality. Antimicrob. Agents Chemother. 2005, 49, 3640–3645. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.; Micek, S.; Hampton, N.; Doherty, J.A.; Kumar, A. Septic shock attributed to Candida infection: Importance of empiric therapy and source control. Clin. Infect. Dis. 2012, 54, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Firacative, C. Invasive fungal disease in humans: Are we aware of the real impact? Mem. Inst. Oswaldo Cruz 2020, 115, e200430. [Google Scholar] [CrossRef] [PubMed]
- Gaffi—Global Action Fund for Fungal Infections. Available online: https://www.gaffi.org (accessed on 3 February 2023).
- Burden of Fungal Diseases in the United States. Fungal Diseases. CDC. Available online: https://www.cdc.gov/fungal/cdc-and-fungal/burden.html (accessed on 3 February 2023).
- Benedict, K.; Jackson, B.R.; Chiller, T.; Beer, K.D. Estimation of Direct Healthcare Costs of Fungal Diseases in the United States. Clin. Infect. Dis. 2019, 68, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Bitar, D.; Lortholary, O.; Le Strat, Y.; Nicolau, J.; Coignard, B.; Tattevin, P.; Che, D.; Dromer, F. Population-based analysis of invasive fungal infections, France, 2001-2010. Emerg. Infect. Dis. 2014, 20, 1149–1155. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kume, H.; Togano, T.; Kanoh, Y.; Ohto, H. Epidemiology of visceral mycoses in autopsy cases in Japan: The data from 1989 to 2009 in the Annual of Pathological Autopsy Cases in Japan. Med. Mycol. 2013, 51, 522–526. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Chatterjee, S.S.; Das, A.; Shivaprakash, M.R. Invasive aspergillosis in developing countries. Med. Mycol. 2011, 49, S35–S47. [Google Scholar] [CrossRef]
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive fungal infections among organ transplant recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J.; Ito, J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Levitz, S.M. Tackling human fungal infections. Science 2012, 336, 647. [Google Scholar] [CrossRef]
- Wauters, J.; Baar, I.; Meersseman, P.; Meersseman, W.; Dams, K.; De Paep, R.; Lagrou, K.; Wilmer, A.; Jorens, P.; Hermans, G. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: A retrospective study. Intensive Care Med. 2012, 38, 1761–1768. [Google Scholar] [CrossRef]
- Schauwvlieghe, A.F.A.D.; Rijnders, B.J.A.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van de Veerdonk, F.L.; Gommers, D.; et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir. Med. 2018, 6, 782–792. [Google Scholar] [CrossRef]
- Van de Veerdonk, F.L.; Kolwijck, E.; Lestrade, P.P.; Hodiamont, C.J.; Rijnders, B.J.; van Paassen, J.; Haas, P.J.; Oliveira Dos Santos, C.; Kampinga, G.A.; Verweij, P.E. Influenza-Associated Aspergillosis in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2017, 196, 524–527. [Google Scholar] [CrossRef]
- Helleberg, M.; Steensen, M.; Arendrup, M.C. Invasive aspergillosis in patients with severe COVID-19 pneumonia. Clin. Microbiol. Infect. 2021, 27, 147–148. [Google Scholar] [CrossRef]
- Alanio, A.; Dellière, S.; Fodil, S.; Bretagne, S.; Mégarbane, B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir. Med. 2020, 8, e48–e49. [Google Scholar] [CrossRef]
- Van Arkel, A.L.E.; Rijpstra, T.A.; Belderbos, H.N.A.; van Wijngaarden, P.; Verweij, P.E.; Bentvelsen, R.G. COVID-19-associated Pulmonary Aspergillosis. Am. J. Respir. Crit. Care Med. 2020, 202, 132–135. [Google Scholar] [CrossRef]
- Koehler, P.; Cornely, O.A.; Böttiger, B.W.; Dusse, F.; Eichenauer, D.A.; Fuchs, F.; Hallek, M.; Jung, N.; Klein, F.; Persigehl, T.; et al. COVID-19 associated pulmonary aspergillosis. Mycoses 2020, 63, 528–534. [Google Scholar] [CrossRef]
- Blaize, M.; Mayaux, J.; Nabet, C.; Lampros, A.; Marcelin, A.G.; Thellier, M.; Piarroux, R.; Demoule, A.; Fekkar, A. Fatal Invasive Aspergillosis and Coronavirus Disease in an Immunocompetent Patient. Emerg. Infect. Dis. 2020, 26, 1636–1637. [Google Scholar] [CrossRef]
- Lescure, F.X.; Bouadma, L.; Nguyen, D.; Parisey, M.; Wicky, P.H.; Behillil, S.; Gaymard, A.; Bouscambert-Duchamp, M.; Donati, F.; Le Hingrat, Q.; et al. Clinical and virological data of the first cases of COVID-19 in Europe: A case series. Lancet Infect. Dis. 2020, 20, 697–706. [Google Scholar] [CrossRef]
- White, P.L.; Dhillon, R.; Cordey, A.; Hughes, H.; Faggian, F.; Soni, S.; Pandey, M.; Whitaker, H.; May, A.; Morgan, M.; et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin. Infect. Dis. 2021, 73, e1634–e1644. [Google Scholar] [CrossRef]
- Machado, M.; Valerio, M.; Álvarez-Uría, A.; Olmedo, M.; Veintimilla, C.; Padilla, B.; De la Villa, S.; Guinea, J.; Escribano, P.; Ruiz-Serrano, M.J.; et al. Invasive pulmonary aspergillosis in the COVID-19 era: An expected new entity. Mycoses 2021, 64, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Brüggemann, R.J.M.; Wauters, J.; Rijnders, B.J.A.; Chiller, T.; van de Veerdonk, F.L. Influenza Coinfection: Be(a)ware of Invasive Aspergillosis. Clin. Infect. Dis. 2020, 70, 349–350. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.H.; Chan, K.S.; Yang, C.C.; Tan, C.K.; Chuang, Y.C.; Yu, W.L. Higher mortality of severe influenza patients with probable aspergillosis than those with and without other coinfections. J. Formos. Med. Assoc. 2017, 116, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Salmanton-García, J.; Sprute, R.; Stemler, J.; Bartoletti, M.; Dupont, D.; Valerio, M.; Garcia-Vidal, C.; Falces-Romero, I.; Machado, M.; de la Villa, S.; et al. COVID-19–Associated Pulmonary Aspergillosis, March–August 2020. Emerg. Infect. Di.s 2021, 27, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Rijnders, B.J.A.; Brüggemann, R.J.M.; Azoulay, E.; Bassetti, M.; Blot, S.; Calandra, T.; Clancy, C.J.; Cornely, O.A.; Chiller, T.; et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: An expert opinion. Intensive Care Med. 2020, 46, 1524–1535. [Google Scholar] [CrossRef]
- Lamoth, F.; Lewis, R.E.; Walsh, T.J.; Kontoyiannis, D.P. Navigating the uncertainties of COVID-19 associated aspergillosis (CAPA): A comparison with influenza associated aspergillosis (IAPA). J. Infect. Dis. 2021, 26, 163. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Lahmer, T.; Kriescher, S.; Herner, A.; Rothe, K.; Spinner, C.D.; Schneider, J.; Mayer, U.; Neuenhahn, M.; Hoffmann, D.; Geisler, F.; et al. Invasive pulmonary aspergillosis in critically ill patients with severe COVID-19 pneumonia: Results from the prospective AspCOVID-19 study. PLoS ONE 2021, 16, e0238825. [Google Scholar] [CrossRef]
- Thompson Iii, G.R.; Cornely, O.A.; Pappas, P.G.; Patterson, T.F.; Hoenigl, M.; Jenks, J.D.; Clancy, C.J.; Nguyen, M.H. Invasive Aspergillosis as an Under-recognized Superinfection in COVID-19. Open Forum Infect. Dis. 2020, 7, ofaa242. [Google Scholar] [CrossRef]
- Russell, C.D.; Millar, J.E.; Baillie, J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020, 395, 473–475. [Google Scholar] [CrossRef]
- Cox, M.J.; Loman, N.; Bogaert, D.; O’Grady, J. Co-infections: Potentially lethal and unexplored in COVID-19. Lancet Microbe 2020, 1, e11. [Google Scholar] [CrossRef]
- Rutsaert, L.; Steinfort, N.; Van Hunsel, T.; Bomans, P.; Naesens, R.; Mertes, H.; Dits, H.; Van Regenmortel, N. COVID-19-associated invasive pulmonary aspergillosis. Ann. Intensive Care 2020, 10, 71. [Google Scholar] [CrossRef]
- Armstrong-James, D.; Youngs, J.; Bicanic, T.; Abdolrasouli, A.; Denning, D.W.; Johnson, E.; Mehra, V.; Pagliuca, T.; Patel, B.; Rhodes, J.; et al. Confronting and mitigating the risk of COVID-19 associated pulmonary aspergillosis. Eur. Respir. J. 2020, 56, 2002554. [Google Scholar] [CrossRef]
- Maertens, J.A.; Blennow, O.; Duarte, R.F.; Muñoz, P. The current management landscape: Aspergillosis. J. Antimicrob. Chemother. 2016, 71, ii23–ii29. [Google Scholar] [CrossRef]
- Singh, D.; Mathioudakis, A.G.; Higham, A. Chronic obstructive pulmonary disease and COVID-19: Interrelationships. Curr. Opin. Pulm. Med. 2022, 28, 76–83. [Google Scholar] [CrossRef]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 2020, 323, 1775–1776. [Google Scholar] [CrossRef]
- Trof, R.J.; Beishuizen, A.; Debets-Ossenkopp, Y.J.; Girbes, A.R.; Groeneveld, A.B. Management of invasive pulmonary aspergillosis in non-neutropenic critically ill patients. Intensive Care Med. 2007, 33, 1694–1703. [Google Scholar] [CrossRef]
- Bartoletti, M.; Pascale, R.; Cricca, M.; Rinaldi, M.; Maccaro, A.; Bussini, L.; Fornaro, G.; Tonetti, T.; Pizzilli, G.; Francalanci, E.; et al. Epidemiology of Invasive Pulmonary Aspergillosis among Intubated Patients with COVID-19: A Prospective Study. Clin. Infect. Dis. 2021, 73, e3606–e3614. [Google Scholar] [CrossRef]
- Vanderbeke, L.; Spriet, I.; Breynaert, C.; Rijnders, B.J.A.; Verweij, P.E.; Wauters, J. Invasive pulmonary aspergillosis complicating severe influenza: Epidemiology, diagnosis and treatment. Curr. Opin. Infect. Dis. 2018, 31, 471–480. [Google Scholar] [CrossRef]
- Wang, H.; Ding, Y.; Li, X.; Yang, L.; Zhang, W.; Kang, W. Fatal aspergillosis in a patient with SARS who was treated with corticosteroids. N. Engl. J. Med. 2003, 349, 507–508. [Google Scholar] [CrossRef]
- Angus, D.C.; Derde, L.; Al-Beidh, F.; Annane, D.; Arabi, Y.; Beane, A.; van Bentum-Puijk, W.; Berry, L.; Bhimani, Z.; Bonten, M.; et al. Effect of Hydrocortisone on Mortality and Organ Support in Patients with Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA 2020, 324, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Tomazini, B.M.; Maia, I.S.; Cavalcanti, A.B.; Berwanger, O.; Rosa, R.G.; Veiga, V.C.; Avezum, A.; Lopes, R.D.; Bueno, F.R.; Silva, M.V.A.O.; et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients with Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA 2020, 324, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Dequin, P.F.; Heming, N.; Meziani, F.; Plantefève, G.; Voiriot, G.; Badié, J.; François, B.; Aubron, C.; Ricard, J.D.; Ehrmann, S.; et al. Effect of Hydrocortisone on 21-Day Mortality or Respiratory Support Among Critically Ill Patients With COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24, e1–e38. [Google Scholar] [CrossRef]
- Hage, C.A.; Carmona, E.M.; Epelbaum, O.; Evans, S.E.; Gabe, L.M.; Haydour, Q.; Knox, K.S.; Kolls, J.K.; Murad, M.H.; Wengenack, N.L.; et al. Microbiological Laboratory Testing in the Diagnosis of Fungal Infections in Pulmonary and Critical Care Practice. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2019, 200, 535–550. [Google Scholar] [CrossRef]
- Patterson, T.F.; Thompson, G.R. 3rd.; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Blot, S.I.; Taccone, F.S.; Van den Abeele, A.M.; Bulpa, P.; Meersseman, W.; Brusselaers, N.; Dimopoulos, G.; Paiva, J.A.; Misset, B.; Rello, J.; et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am. J. Respir. Crit. Care Med. 2012, 186, 56–64. [Google Scholar] [CrossRef]
- Thornton, C.; Johnson, G.; Agrawal, S. Detection of invasive pulmonary aspergillosis in haematological malignancy patients by using lateral-flow technology. J. Vis. Exp. 2012, 61, 3721. [Google Scholar] [CrossRef]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [CrossRef]
- Hope, W.W.; Walsh, T.J.; Denning, D.W. Laboratory diagnosis of invasive aspergillosis. Lancet Infect. Dis. 2005, 5, 609–622. [Google Scholar] [CrossRef]
- Thornton, C.R. Detection of invasive aspergillosis. Adv. Appl. Microbiol. 2010, 70, 187–216. [Google Scholar] [CrossRef]
- Pickering, J.W.; Sant, H.W.; Bowles, C.A.P.; Roberts, W.L.; Woods, G.L. Evaluation of a (1->3)-beta-D-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 2005, 43, 5957–5962. [Google Scholar] [CrossRef]
- Meersseman, W.; Lagrou, K.; Maertens, J.; Wilmer, A.; Hermans, G.; Vanderschueren, S.; Spriet, I.; Verbeken, E.; Van Wijngaerden, E. Galactomannan in bronchoalveolar lavage fluid: A tool for diagnosing aspergillosis in intensive care unit patients. Am. J. Respir. Crit. Care Med. 2008, 177, 27–34. [Google Scholar] [CrossRef]
- Verweij, P.E.; Gangneux, J.P.; Bassetti, M.; Brüggemann, R.J.M.; Cornely, O.A.; Koehler, P.; Lass-Flörl, C.; van de Veerdonk, F.L.; Chakrabarti, A.; Hoenigl, M.; et al. Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe 2020, 1, e53–e55. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Thornton, C.R.; Najvar, L.K.; Kirkpatrick, W.R.; Bocanegra, R.; Patterson, T.F. Comparison of Lateral Flow Technology and Galactomannan and (1→3)-β-d-Glucan Assays for Detection of Invasive Pulmonary Aspergillosis. Clin. Vaccine Immunol. 2009, 16, 1844–1846. [Google Scholar] [CrossRef]
- Heldt, S.; Hoenigl, M. Lateral Flow Assays for the Diagnosis of Invasive Aspergillosis: Current Status. Curr. Fungal Infect. Rep. 2017, 11, 45–51. [Google Scholar] [CrossRef]
- Hoenigl, M.; Eigl, S.; Heldt, S.; Duettmann, W.; Thornton, C.; Prattes, J. Clinical evaluation of the newly formatted lateral-flow device for invasive pulmonary aspergillosis. Mycoses 2018, 61, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Wahidi, M.M.; Lamb, C.; Murgu, S.; Musani, A.; Shojaee, S.; Sachdeva, A.; Maldonado, F.; Mahmood, K.; Kinsey, M.; Sethi, S.; et al. American Association for Bronchology and Interventional Pulmonology (AABIP) Statement on the Use of Bronchoscopy and Respiratory Specimen Collection in Patients with Suspected or Confirmed COVID-19 Infection. J. Bronchol. Interv. Pulmonol. 2020, 27, e52–e54. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.; Abraham, W.R. Volatile sesquiterpenes from fungi: What are they good for? Phytochem. Rev. 2012, 11, 15–37. [Google Scholar] [CrossRef]
- Bassetti, M.; Peghin, M.; Vena, A. Challenges and Solution of Invasive Aspergillosis in Non-neutropenic Patients: A Review. Infect. Dis. Ther. 2018, 7, 17–27. [Google Scholar] [CrossRef]
- Koo, S.; Thomas, H.R.; Daniels, S.D.; Lynch, R.C.; Fortier, S.M.; Shea, M.M.; Rearden, P.; Comolli, J.C.; Baden, L.R.; Marty, F.M. A Breath Fungal Secondary Metabolite Signature to Diagnose Invasive Aspergillosis. Clin. Infect. Dis. 2014, 59, 1733–1740. [Google Scholar] [CrossRef]
- Mercier, T.; Reséndiz Sharpe, A.; Waumans, D.; Desmet, K.; Lagrou, K.; Maertens, J. Gliotoxin and bis(methylthio)gliotoxin are not reliable as biomarkers of invasive aspergillosis. Mycoses 2019, 62, 945–948. [Google Scholar] [CrossRef]
- Zhou, W.; Li, H.; Zhang, Y.; Huang, M.; He, Q.; Li, P.; Zhang, F.; Shi, Y.; Su, X. Diagnostic Value of Galactomannan Antigen Test in Serum and Bronchoalveolar Lavage Fluid Samples from Patients with Nonneutropenic Invasive Pulmonary Aspergillosis. J. Clin. Microbiol. 2017, 55, 2153–2161. [Google Scholar] [CrossRef]
- Rickerts, V.; Mousset, S.; Lambrecht, E.; Tintelnot, K.; Schwerdtfeger, R.; Presterl, E.; Jacobi, V.; Just-Nübling, G.; Bialek, R. Comparison of histopathological analysis, culture, and polymerase chain reaction assays to detect invasive mold infections from biopsy specimens. Clin. Infect. Dis. 2007, 44, 1078–1083. [Google Scholar] [CrossRef]
- Lewis, R.E.; Kontoyiannis, D.P. Invasive aspergillosis in glucocorticoid-treated patients. Med. Mycol. 2009, 47, S271–S281. [Google Scholar] [CrossRef]
- Zou, M.; Tang, L.; Zhao, S.; Zhao, Z.; Chen, L.; Chen, P.; Huang, Z.; Li, J.; Chen, L.; Fan, X. Systematic review and meta-analysis of detecting galactomannan in bronchoalveolar lavage fluid for diagnosing invasive aspergillosis. PLoS ONE 2012, 7, e43347. [Google Scholar] [CrossRef]
- Guo, Y.L.; Chen, Y.Q.; Wang, K.; Qin, S.M.; Wu, C.; Kong, J.L. Accuracy of BAL galactomannan in diagnosing invasive aspergillosis: A bivariate metaanalysis and systematic review. Chest 2010, 138, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Eigl, S.; Prattes, J.; Reinwald, M.; Thornton, C.R.; Reischies, F.; Spiess, B.; Neumeister, P.; Zollner-Schwetz, I.; Raggam, R.B.; Flick, H.; et al. Influence of mould-active antifungal treatment on the performance of the Aspergillus-specific bronchoalveolar lavage fluid lateral-flow device test. Int. J. Antimicrob. Agents 2015, 46, 401–405. [Google Scholar] [CrossRef] [PubMed]
- De Heer, K.; Gerritsen, M.G.; Visser, C.E.; Leeflang, M.M. Galactomannan detection in broncho-alveolar lavage fluid for invasive aspergillosis in immunocompromised patients. Cochrane Database Syst. Rev. 2019, 5, CD012399. [Google Scholar] [CrossRef] [PubMed]
- Theel, E.S.; Jespersen, D.J.; Iqbal, S.; Bestrom, J.E.; Rollins, L.O.; Misner, L.J.; Markley, B.J.; Mandrekar, J.; Baddour, L.M.; Limper, A.H.; et al. Detection of (1, 3)-β-D-glucan in bronchoalveolar lavage and serum samples collected from immunocompromised hosts. Mycopathologia 2013, 175, 33–41. [Google Scholar] [CrossRef] [PubMed]
- White, P.L.; Wiederhold, N.P.; Loeffler, J.; Najvar, L.K.; Melchers, W.; Herrera, M.; Bretagne, S.; Wickes, B.; Kirkpatrick, W.R.; Barnes, R.A.; et al. Comparison of Nonculture Blood-Based Tests for Diagnosing Invasive Aspergillosis in an Animal Model. J. Clin. Microbiol. 2016, 54, 960–966. [Google Scholar] [CrossRef]
- White, P.L.; Wingard, J.R.; Bretagne, S.; Löffler, J.; Patterson, T.F.; Slavin, M.A.; Barnes, R.A.; Pappas, P.G.; Donnelly, J.P. Aspergillus Polymerase Chain Reaction: Systematic Review of Evidence for Clinical Use in Comparison with Antigen Testing. Clin. Infect. Dis. 2015, 61, 1293–1303. [Google Scholar] [CrossRef]
- Arvanitis, M.; Ziakas, P.D.; Zacharioudakis, I.M.; Zervou, F.N.; Caliendo, A.M.; Mylonakis, E. PCR in diagnosis of invasive aspergillosis: A meta-analysis of diagnostic performance. J. Clin. Microbiol. 2014, 52, 3731–3742. [Google Scholar] [CrossRef]
- Arvanitis, M.; Anagnostou, T.; Mylonakis, E. Galactomannan and Polymerase Chain Reaction-Based Screening for Invasive Aspergillosis Among High-Risk Hematology Patients: A Diagnostic Meta-analysis. Clin. Infect. Dis. 2015, 61, 1263–1272. [Google Scholar] [CrossRef]
- Mengoli, C.; Cruciani, M.; Barnes, R.A.; Loeffler, J.; Donnelly, J.P. Use of PCR for diagnosis of invasive aspergillosis: Systematic review and meta-analysis. Lancet Infect. Dis. 2009, 9, 89–96. [Google Scholar] [CrossRef]
- Hoenigl, M.; Prattes, J.; Spiess, B.; Wagner, J.; Prueller, F.; Raggam, R.B.; Posch, V.; Duettmann, W.; Hoenigl, K.; Wölfler, A.; et al. Performance of galactomannan, beta-d-glucan, Aspergillus lateral-flow device, conventional culture, and PCR tests with bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis. J. Clin. Microbiol. 2014, 52, 2039–2045. [Google Scholar] [CrossRef]
- Pan, Z.; Fu, M.; Zhang, J.; Zhou, H.; Fu, Y.; Zhou, J. Diagnostic accuracy of a novel lateral-flow device in invasive aspergillosis: A meta-analysis. J. Med. Microbiol. 2015, 64, 702–707. [Google Scholar] [CrossRef]
- Prattes, J.; Flick, H.; Prüller, F.; Koidl, C.; Raggam, R.B.; Palfner, M.; Eigl, S.; Buzina, W.; Zollner-Schwetz, I.; Thornton, C.R.; et al. Novel tests for diagnosis of invasive aspergillosis in patients with underlying respiratory diseases. Am. J. Respir. Crit. Care Med. 2014, 190, 922–929. [Google Scholar] [CrossRef]
- Acharige, M.J.T.; Koshy, S.; Ismail, N.; Aloum, O.; Jazaerly, M.; Astudillo, C.L.; Koo, S. Breath-based diagnosis of fungal infections. J. Breath Res. 2018, 12, 027108. [Google Scholar] [CrossRef]
- Vidal-García, M.; Domingo, M.P.; De Rueda, B.; Roc, L.; Delgado, M.P.; Revillo, M.J.; Pardo, J.; Gálvez, E.M.; Rezusta, A. Clinical validity of bis(methylthio)gliotoxin for the diagnosis of invasive aspergillosis. Appl. Microbiol. Biotechnol. 2016, 100, 2327–2334. [Google Scholar] [CrossRef]
- Vidal-García, M.; Sánchez-Chueca, P.; Domingo, M.P.; Ballester, C.; Roc, L.; Ferrer, I.; Revillo, M.J.; Pardo, J.; Gálvez, E.M.; Rezusta, A. Disseminated aspergillosis in an immunocompetent patient with detectable bis(methylthio)gliotoxin and negative galactomannan. Rev. Iberoam. Micol. 2017, 34, 49–52. [Google Scholar] [CrossRef]
- Greene, R.E.; Schlamm, H.T.; Oestmann, J.W.; Stark, P.; Durand, C.; Lortholary, O.; Wingard, J.R.; Herbrecht, R.; Ribaud, P.; Patterson, T.F.; et al. Imaging findings in acute invasive pulmonary aspergillosis: Clinical significance of the halo sign. Clin. Infect. Dis. 2007, 44, 373–379. [Google Scholar] [CrossRef]
- Meersseman, W.; Lagrou, K.; Maertens, J.; Van Wijngaerden, E. Invasive aspergillosis in the intensive care unit. Clin. Infect. Dis. 2007, 45, 205–216. [Google Scholar] [CrossRef]
- Jenks, J.D.; Mehta, S.R.; Hoenigl, M. Broad spectrum triazoles for invasive mould infections in adults: Which drug and when? Med. Mycol. 2019, 57, S168–S178. [Google Scholar] [CrossRef]
- Barchiesi, F.; Santinelli, A.; Biscotti, T.; Greganti, G.; Giannini, D.; Manso, E. Delay of antifungal therapy influences the outcome of invasive aspergillosis in experimental models of infection. J. Antimicrob. Chemother. 2016, 71, 2230–2233. [Google Scholar] [CrossRef]
- Russo, A.; Tiseo, G.; Falcone, M.; Menichetti, F. Pulmonary Aspergillosis: An Evolving Challenge for Diagnosis and Treatment. Infect. Dis. Ther. 2020, 9, 511–524. [Google Scholar] [CrossRef]
- Silva, L.N.; de Mello, T.P.; de Souza Ramos, L.; Branquinha, M.H.; Roudbary, M.; Dos Santos, A.L.S. Fungal Infections in COVID-19-Positive Patients: A Lack of Optimal Treatment Options. Curr. Top. Med. Chem. 2020, 20, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Carvalho, A.; van de Veerdonk, F.L.; Jenks, J.D.; Koehler, P.; Krause, R.; Cornely, O.A.; Perlin, D.S.; Lass-Flörl, C.; Hoenigl, M. COVID-19 Associated Pulmonary Aspergillosis (CAPA)—From Immunology to Treatment. J. Fungi 2020, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Baddley, J.W.; Stephens, J.M.; Ji, X.; Gao, X.; Schlamm, H.T.; Tarallo, M. Aspergillosis in Intensive Care Unit (ICU) patients: Epidemiology and economic outcomes. BMC Infect. Dis. 2013, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, M.P.; Toussaint, E.; Denis, J.; Herbrecht, R. New pharmacological opportunities for the treatment of invasive mould diseases. J. Antimicrob. Chemother. 2017, 72, i48–i58. [Google Scholar] [CrossRef]
- McCreary, E.K.; Pogue, J.M. Coronavirus Disease 2019 Treatment: A Review of Early and Emerging Options. Open Forum Infect. Dis. 2020, 7, ofaa105. [Google Scholar] [CrossRef]
- Brüggemann, R.J.; Alffenaar, J.W.; Blijlevens, N.M.; Billaud, E.M.; Kosterink, J.G.; Verweij, P.E.; Burger, D.M. Clinical Relevance of the Pharmacokinetic Interactions of Azole Antifungal Drugs with Other Coadministered Agents. Clin. Infect. Dis. 2009, 48, 1441–1458. [Google Scholar] [CrossRef]
- Moriyama, B.; Kadri, S.; Henning, S.A.; Danner, R.L.; Walsh, T.J.; Penzak, S.R. Therapeutic Drug Monitoring and Genotypic Screening in the Clinical Use of Voriconazole. Curr. Fungal Infect. Rep. 2015, 9, 74–87. [Google Scholar] [CrossRef]
- Job, K.M.; Olson, J.; Stockmann, C.; Constance, J.E.; Enioutina, E.Y.; Rower, J.E.; Linakis, M.W.; Balch, A.H.; Yu, T.; Liu, X.; et al. Pharmacodynamic studies of voriconazole: Informing the clinical management of invasive fungal infections. Expert Rev. Anti-Infect. Ther. 2016, 14, 731–746. [Google Scholar] [CrossRef]
- Ashbee, H.R.; Barnes, R.A.; Johnson, E.M.; Richardson, M.D.; Gorton, R.; Hope, W.W. Therapeutic drug monitoring (TDM) of antifungal agents: Guidelines from the British Society for Medical Mycology. J. Antimicrob. Chemother. 2014, 69, 1162–1176. [Google Scholar] [CrossRef]
- Wilson, D.T.; Dimondi, V.P.; Johnson, S.W.; Jones, T.M.; Drew, R.H. Role of isavuconazole in the treatment of invasive fungal infections. Ther. Clin. Risk Mana.g 2016, 12, 1197–1206. [Google Scholar] [CrossRef]
- Natesan, S.K.; Chandrasekar, P.H. Isavuconazole for the treatment of invasive aspergillosis and mucormycosis: Current evidence, safety, efficacy, and clinical recommendations. Infect. Drug Resist. 2016, 9, 291–300. [Google Scholar] [CrossRef]
- Puelles, V.G.; Lütgehetmann, M.; Lindenmeyer, M.T.; Sperhake, J.P.; Wong, M.N.; Allweiss, L.; Chilla, S.; Heinemann, A.; Wanner, N.; Liu, S.; et al. Multiorgan and Renal Tropism of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 590–592. [Google Scholar] [CrossRef]
- Pesaresi, M.; Pirani, F.; Tagliabracci, A.; Valsecchi, M.; Procopio, A.D.; Busardò, F.P.; Graciotti, L. SARS-CoV-2 identification in lungs, heart and kidney specimens by transmission and scanning electron microscopy. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5186–5188. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Aruanno, M.; Glampedakis, E.; Lamoth, F. Echinocandins for the Treatment of Invasive Aspergillosis: From Laboratory to Bedside. Antimicrob. Agents Chemother. 2019, 63, e00399-19. [Google Scholar] [CrossRef]
- Kupferschmidt, K. New drugs target growing threat of fatal fungi. Science 2019, 366, 407. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Locke, J.B.; Daruwala, P.; Bartizal, K. Rezafungin (CD101) demonstrates potent in vitro activity against Aspergillus, including azole-resistant Aspergillus fumigatus isolates and cryptic species. J. Antimicrob. Chemother. 2018, 73, 3063–3067. [Google Scholar] [CrossRef]
- Aldrees, A.; Ghonem, L.; Almajid, F.; Barry, M.; Mayet, A.; Almohaya, A.M. Evaluating the Inappropriate Prescribing and Utilization of Caspofungin, a Four-Year Analysis at a Teaching Hospital in Saudi Arabia. Antibiotics 2021, 10, 1498. [Google Scholar] [CrossRef]
- Estella, Á.; Recuerda Núñez, M.; Lagares, C.; Gracia Romero, M.; Torres, E.; Alados Arboledas, J.C.; Antón Escors, Á.; González García, C.; Sandar Núñez, D.; López Prieto, D.; et al. Anticipatory Antifungal Treatment in Critically Ill Patients with SARS-CoV-2 Pneumonia. J. Fungi 2023, 9, 288. [Google Scholar] [CrossRef]
- Verweij, P.E.; Brüggemann, R.J.M.; Azoulay, E.; Bassetti, M.; Blot, S.; Buil, J.B.; Calandra, T.; Chiller, T.; Clancy, C.J.; Cornely, O.A.; et al. Taskforce report on the diagnosis and clinical management of COVID-19 associated pulmonary aspergillosis. Intensive Care Med. 2021, 47, 819–834. [Google Scholar] [CrossRef]
- Van Daele, R.; Bekkers, B.; Lindfors, M.; Broman, L.M.; Schauwvlieghe, A.; Rijnders, B.; Hunfeld, N.G.M.; Juffermans, N.P.; Taccone, F.S.; Sousa, C.A.C.; et al. A large retrospective assessment of voriconazole exposure in patients treated with extracorporeal membrane oxygenation. Microorganisms 2021, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Reizine, F.; Pinceaux, K.; Lederlin, M.; Autier, B.; Guegan, H.; Gacouin, A.; Luque-Paz, D.; Boglione-Kerrien, C.; Bacle, A.; Le Daré, B.; et al. Influenza- and COVID-19-Associated Pulmonary Aspergillosis: Are the Pictures Different? J. Fungi 2021, 7, 388. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.J.; Gao, K.Q.; Huang, P.H.; Guo, R.; Zuo, X.C.; Xia, Q.; Hu, S.Y.; Yu, Z.; Xie, Y.L. Interactive Effects of Glucocorticoids and Cytochrome P450 Polymorphisms on the Plasma Trough Concentrations of Voriconazole. Front. Pharmacol. 2021, 12, 666296. [Google Scholar] [CrossRef] [PubMed]
- Van de Veerdonk, F.L.; Giamarellos-Bourboulis, E.; Pickkers, P.; Derde, L.; Leavis, H.; van Crevel, R.; Engel, J.J.; Wiersinga, W.J.; Vlaar, A.P.J.; Shankar-Hari, M.; et al. A guide to immunotherapy for COVID-19. Nat. Med. 2022, 28, 39–50. [Google Scholar] [CrossRef]

| Tests | Features | Diagnostic Value | Turnaround | Pitfalls |
|---|---|---|---|---|
| Conventional microscopic examination [36,49,50,51,69,70] | Availability. Simplicity. Low cost. | Suboptimal, low to moderate sensitivity and predictive value. | Rapid. | Challenging to differentiate between infection and colonization. May reflect airway colonization. |
| Respiratory sample cultures [49,50,51,56,70] | Simplicity. Low cost. Identification of species. Antifungal susceptibility testing. | Suboptimal, low to moderate sensitivity and predictive value. | Prolonged. | Challenging to differentiate between infection and colonization. |
| Galactomannan (GM) in biologic fluids [36,49,51,55,69,71,72,73,74,75] | Serum: Low or moderate sensitivity depending on the index cut-off used. Moderate specificity. Better performance in neutropenic than in non-neutropenic patients. BAL: Moderate or high sensitivity and high specificity of 81–96.6% depending on the optical density index cut-off used, sensitivity exceeds 70% in most studies. Raising the cutoff improves test specificity without compromising sensitivity. High NPV, moderate or high PPV. | Variable. | Variable performance. BAL: Optimal threshold has not been determined; sensitivity may be reduced in the presence of antifungals. | |
| Serum 1-3-b-D-glucan (BDG) assay [36,49,51,66,76] | Low or moderate sensitivity (49.6–80%), good specificity (82–98.9%), acceptable PPV (83.5%), high NPV (89–94.6%) (useful to exclude diagnosis rather than confirm it). | Variable. | False-positive results (b-lactam antibiotics, human blood products, immunoglobulin, albumin plasma, cellulose hemodialysis membranes, bacterial bloodstream infections, e.g., Pseudomonas aeruginosa) | |
| PCR-based methods [36,49,51,70,77,78,79,80,81,82] | High cost. Not affected by the immune status of the patients. Evaluation of phenotypes of strains. | Heterogeneity of results. High NPV. Two positive consecutive results have high specificity and high positive likelihood ratio, single negative PCR result has high NPV. High sensitivity in combination with other fungal biomarkers in serum (either GM or BDG) or in BAL and along with GM and/or LFD test. | Rapid. | Requires further clinical standardization. Potential for contamination due to the environmental ubiquity of fungal nucleic acids. |
| Aspergillus-specific immuno-chromatographic lateral flow device (LFD) test [36,49,51,63,66,74,83] | Acceptable sensitivity, specificity, moderate PPV, high NPV (especially in combination with BAL GM) [66,84]. | Rapid. | Requires further clinical evaluation. Sensitivity of the BAL LFD assay may be reduced in the presence of antifungal treatment. | |
| Novel assays: volatile organic compounds (VOC) assays, Gliotoxin (GT), bis(methylthio)gliotoxin (bmGT) assays [67,68,85,86,87] | High sensitivity and specificity. bmGT presents higher sensitivity and PPV than GM and similar specificity and NPV. Importantly, the combination of GM and bmGT increased the PPV (100%) and NPV (97.5%) of the individual biomarkers. | Rapid. | Requires further clinical evaluation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsotsolis, S.; Kotoulas, S.-C.; Lavrentieva, A. Invasive Pulmonary Aspergillosis in Coronavirus Disease 2019 Patients Lights and Shadows in the Current Landscape. Adv. Respir. Med. 2023, 91, 185-202. https://doi.org/10.3390/arm91030016
Tsotsolis S, Kotoulas S-C, Lavrentieva A. Invasive Pulmonary Aspergillosis in Coronavirus Disease 2019 Patients Lights and Shadows in the Current Landscape. Advances in Respiratory Medicine. 2023; 91(3):185-202. https://doi.org/10.3390/arm91030016
Chicago/Turabian StyleTsotsolis, Stavros, Serafeim-Chrysovalantis Kotoulas, and Athina Lavrentieva. 2023. "Invasive Pulmonary Aspergillosis in Coronavirus Disease 2019 Patients Lights and Shadows in the Current Landscape" Advances in Respiratory Medicine 91, no. 3: 185-202. https://doi.org/10.3390/arm91030016