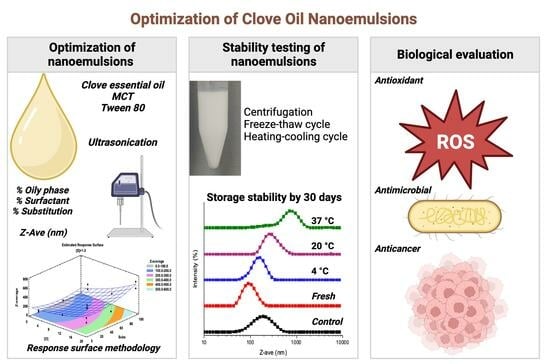
Optimization of Clove Oil Nanoemulsions: Evaluation of Antioxidant, Antimicrobial, and Anticancer Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Nanoemulsions by Ultrasonication
2.2.2. Emulsion Droplet Size Distribution
2.2.3. Stability Testing of Nanoemulsions
2.2.4. Antioxidant Activity
DPPH Radical Capture Assay
Determination of Total Phenol Content
2.2.5. Antimicrobial Activity
Well Diffusion Assay
Minimal Inhibitory Concentration (MIC)
Minimum Bactericidal Concentration (MCB)
2.2.6. Anticancer Activity
Cell Lines and Culturing
Cytotoxicity Assay
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Conditions to Produce CEO–NEs
3.2. Characterization and Evaluation of the Optimized Nanoemulsion
3.3. Antioxidant Activity
3.4. Antimicrobial Activity
3.5. Anticancer Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove Essential Oil (Syzygium aromaticum L. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef]
- Franklyne, J.S.; Iyer, S.; Ebenazer, A.; Mukherjee, A.; Chandrasekaran, N. Essential Oil Nanoemulsions: Antibacterial Activity in Contaminated Fruit Juices. Int. J. Food Sci. Technol. 2019, 54, 2802–2810. [Google Scholar] [CrossRef]
- Badr, M.M.; Badawy, M.E.I.; Taktak, N.E.M. Characterization, Antimicrobial Activity, and Antioxidant Activity of the Nanoemulsions of Lavandula Spica Essential Oil and Its Main Monoterpenes. J. Drug Deliv. Sci. Technol. 2021, 65, 102732. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Espinosa-Andrews, H.; Páez-Hernández, G. Optimization of Ultrasonication Curcumin-Hydroxylated Lecithin Nanoemulsions Using Response Surface Methodology. J. Food Sci. Technol. 2020, 57, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Chawla, P.; Arora, S. Development and Evaluation of Iron Microencapsules for Milk Fortification. CyTA-J. Food 2015, 13, 116–123. [Google Scholar] [CrossRef]
- Wan, J.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Physical Properties, Antifungal and Mycotoxin Inhibitory Activities of Five Essential Oil Nanoemulsions: Impact of Oil Compositions and Processing Parameters. Food Chem. 2019, 291, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Influence of Oil Phase Composition on the Antifungal and Mycotoxin Inhibitory Activity of Clove Oil Nanoemulsions. Food Funct. 2018, 9, 2872–2882. [Google Scholar] [CrossRef]
- Chang, Y.; McLandsborough, L.; McClements, D.J. Physicochemical Properties and Antimicrobial Efficacy of Carvacrol Nanoemulsions Formed by Spontaneous Emulsification. J. Agric. Food Chem. 2013, 61, 8906–8913. [Google Scholar] [CrossRef]
- Chang, Y.; McClements, D.J. Optimization of Orange Oil Nanoemulsion Formation by Isothermal Low-Energy Methods: Influence of the Oil Phase, Surfactant, and Temperature. J. Agric. Food Chem. 2014, 62, 2306–2312. [Google Scholar] [CrossRef]
- Páez-Hernández, G.; Mondragón-Cortez, P.; Espinosa-Andrews, H. Developing Curcumin Nanoemulsions by High-Intensity Methods: Impact of Ultrasonication and Microfluidization Parameters. LWT 2019, 111, 291–300. [Google Scholar] [CrossRef]
- Abadi, A.V.M.; Karimi, E.; Oskoueian, E.; Mohammad, G.R.K.S.; Shafaei, N. Chemical Investigation and Screening of Anti-Cancer Potential of Syzygium aromaticum L. Bud (Clove) Essential Oil Nanoemulsion. 3 Biotech 2022, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.S.; Sahu, U.; Karthik, P.; Vendan, S.E. Eugenol Nanoemulsion as Bio-Fumigant: Enhanced Insecticidal Activity against the Rice Weevil, Sitophilus Oryzae Adults. J. Food Sci. Technol. 2023, 60, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Gao, Y.; Yan, W.; Zhang, Z.; Sarker, S.; Yin, Y.; Liu, Q.; Feng, J.; Chen, J. Preparation of Eugenol Nanoemulsions for Antibacterial Activities. RSC Adv. 2022, 12, 3180–3190. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, P.G.; Sengupta, P.; Chicgovinda, P.P.; Rao, P.J. Nanoencapsulation of Clove Oil and Study of Physicochemical Properties, Cytotoxic, Hemolytic, and Antioxidant Activities. J. Food Process Eng. 2021, 44, e13645. [Google Scholar] [CrossRef]
- Wan, J.; Jin, Z.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Clove Oil-in-Water Nanoemulsion: Mitigates Growth of Fusarium Graminearum and Trichothecene Mycotoxin Production during the Malting of Fusarium Infected Barley. Food Chem. 2020, 312, 126120. [Google Scholar] [CrossRef]
- Banerjee, K.; Madhyastha, H.; Rajendra Sandur, V.; Manikandanath, N.T.; Thiagarajan, N.; Thiagarajan, P. Anti-Inflammatory and Wound Healing Potential of a Clove Oil Emulsion. Colloids Surf. B Biointerfaces 2020, 193, 111102. [Google Scholar] [CrossRef] [PubMed]
- da Silva Gündel, S.; Velho, M.C.; Diefenthaler, M.K.; Favarin, F.R.; Copetti, P.M.; de Oliveira Fogaça, A.; Klein, B.; Wagner, R.; Gündel, A.; Sagrillo, M.R.; et al. Basil Oil-Nanoemulsions: Development, Cytotoxicity and Evaluation of Antioxidant and Antimicrobial Potential. J. Drug Deliv. Sci. Technol. 2018, 46, 378–383. [Google Scholar] [CrossRef]
- Sun, H.; Yang, R.; Wang, J.; Yang, X.; Tu, J.; Xie, L.; Li, C.; Lao, Q.; Sun, C. Component-Based Biocompatibility and Safety Evaluation of Polysorbate 80. RSC Adv. 2017, 7, 15127–15138. [Google Scholar] [CrossRef]
- Bu, P.; Ji, Y.; Narayanan, S.; Dalrymple, D.; Cheng, X.; Serajuddin, A.T.M. Assessment of Cell Viability and Permeation Enhancement in Presence of Lipid-Based Self-Emulsifying Drug Delivery Systems Using Caco-2 Cell Model: Polysorbate 80 as the Surfactant. Eur. J. Pharm. Sci. 2017, 99, 350–360. [Google Scholar] [CrossRef]
- Desai, H.H.; Bu, P.; Shah, A.V.; Cheng, X.; Serajuddin, A.T.M. Evaluation of Cytotoxicity of Self-Emulsifying Formulations Containing Long-Chain Lipids Using Caco-2 Cell Model: Superior Safety Profile Compared to Medium-Chain Lipids. J. Pharm. Sci. 2020, 109, 1752–1764. [Google Scholar] [CrossRef]
- Shahavi, M.H.; Hosseini, M.; Jahanshahi, M.; Meyer, R.L.; Darzi, G.N. Clove Oil Nanoemulsion as an Effective Antibacterial Agent: Taguchi Optimization Method. Desalination Water Treat. 2016, 57, 18379–18390. [Google Scholar] [CrossRef]
- Behbahani, B.A.; Noshad, M.; Falah, F. Study of Chemical Structure, Antimicrobial, Cytotoxic and Mechanism of Action of Syzygium Aromaticum Essential Oil on Foodborne Pathogens. Potravin. Slovak J. Food Sci. 2019, 13, 875–883. [Google Scholar] [CrossRef]
- Nirmala, M.J.; Durai, L.; Gopakumar, V.; Nagarajan, R. Anticancer and Antibacterial Effects of a Clove Bud Essential Oil-Based Nanoscale Emulsion System. Int. J. Nanomed. 2019, 14, 6439–6450. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C.M.F.; Rall, V.L.M.; Saeki, M.J.; Fernandes Júnior, A. Inhibitory Effect of Essential Oils against Lactobacillus Rhamnosus and Starter Culture in Fermented Milk during Its Shelf-Life Period. Braz. J. Microbiol. 2012, 43, 1147–1156. [Google Scholar] [CrossRef]
- Ramadan, M.M.; Ali, M.M.; Ghanem, K.Z.; El-Ghorabe, A.H. Essential Oils from Egyptian Aromatic Plants as Antioxidant and Novel Anticancer Agents in Human Cancer Cell Lines. Grasas Y Aceites 2015, 66, e080. [Google Scholar] [CrossRef]
- Najar, B.; Shortrede, J.E.; Pistelli, L.; Buhagiar, J. Chemical Composition and in Vitro Cytotoxic Screening of Sixteen Commercial Essential Oils on Five Cancer Cell Lines. Chem. Biodivers. 2020, 17, e1900478. [Google Scholar] [CrossRef]
- Kouidhi, B.; Zmantar, T.; Bakhrouf, A. Anticariogenic and Cytotoxic Activity of Clove Essential Oil (Eugenia Caryophyllata) against a Large Number of Oral Pathogens. Ann. Microbiol. 2010, 60, 599–604. [Google Scholar] [CrossRef]
- Restu, W.K.; Sampora, Y.; Meliana, Y.; Haryono, A. Effect of Accelerated Stability Test on Characteristics of Emulsion Systems with Chitosan as a Stabilizer. Procedia Chem. 2015, 16, 171–176. [Google Scholar] [CrossRef]
- Gulotta, A.; Saberi, A.H.; Nicoli, M.C.; McClements, D.J. Nanoemulsion-Based Delivery Systems for Polyunsaturated (ω-3) Oils: Formation Using a Spontaneous Emulsification Method. J. Agric. Food Chem. 2014, 62, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Ivanovic, J.; Dimitrijevic-Brankovic, S.; Misic, D.; Ristic, M.; Zizovic, I. Evaluation and Improvement of Antioxidant and Antibacterial Activities of Supercritical Extracts from Clove Buds. J. Funct. Foods 2013, 5, 416–423. [Google Scholar] [CrossRef]
- Wang, H.F.; Yih, K.H.; Yang, C.H.; Huang, K.F. Anti-Oxidant Activity and Major Chemical Component Analyses of Twenty-Six Commercially Available Essential Oils. J. Food Drug Anal. 2017, 25, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Kiokias, S.; Oreopoulou, V. Review on the Antioxidant Activity of Phenolics in o/w Emulsions along with the Impact of a Few Important Factors on Their Interfacial Behaviour. Colloids Interfaces 2022, 6, 79. [Google Scholar] [CrossRef]
- Kiralan, S.S.; Doğu-Baykut, E.; Kittipongpittaya, K.; McClements, D.J.; Decker, E.A. Increased Antioxidant Efficacy of Tocopherols by Surfactant Solubilization in Oil-in-Water Emulsions. J. Agric. Food Chem. 2014, 62, 10561–10566. [Google Scholar] [CrossRef] [PubMed]
- Almajano, M.P.; Delgado, M.E.; Gordon, M.H. Albumin Causes a Synergistic Increase in the Antioxidant Activity of Green Tea Catechins in Oil-in-Water Emulsions. Food Chem. 2007, 102, 1375–1382. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, H.; Feng, Y.; Yu, M.; Xu, Y.; Zhao, Y.; Zheng, B.; Lin, J.; Miao, W.; Zhou, R.; et al. Emulsions Containing Composite (Clove, Oregano, and Cinnamon) Essential Oils: Phase Inversion Preparation, Physicochemical Properties and Antibacterial Mechanism. Food Chem. 2023, 421, 136201. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, S.R.; Bezerra, A.H.; de Sousa Silveira, Z.; Macedo, N.S.; dos Santos Barbosa, C.R.; Muniz, D.F.; Sampaio dos Santos, J.F.; Melo Coutinho, H.D.; Bezerra da Cunha, F.A. Antibacterial Activity of Eugenol on the IS-58 Strain of Staphylococcus Aureus Resistant to Tetracycline and Toxicity in Drosophila Melanogaster. Microb. Pathog. 2022, 164, 105456. [Google Scholar] [CrossRef]
- Lady, J.; Nurcahyanti, A.D.R.; Tjoa, E. Synergistic Effect and Time-Kill Evaluation of Eugenol Combined with Cefotaxime Against Staphylococcus Aureus. Curr. Microbiol. 2023, 80, 244. [Google Scholar] [CrossRef]
- Al-Zereini, W.A.; Al-Trawneh, I.N.; Al-Qudah, M.A.; Tumallah, H.M.; Abudayeh, Z.H.; Hijazin, T. Antibacterial, Antioxidant, and Cytotoxic Activities of Syzygium aromaticum (L.) Merr. & Perry Essential Oil with Identification of Its Chemical Constituents. Z. Fur Naturforschung-Sect. C J. Biosci. 2023, 78, 105–112. [Google Scholar] [CrossRef]
- Radünz, M.; da Trindade, M.L.M.; Camargo, T.M.; Radünz, A.L.; Borges, C.D.; Gandra, E.A.; Helbig, E. Antimicrobial and Antioxidant Activity of Unencapsulated and Encapsulated Clove (Syzygium aromaticum, L.) Essential Oil. Food Chem. 2019, 276, 180–186. [Google Scholar] [CrossRef]
- Falleh, H.; Ben Jemaa, M.; Neves, M.A.; Isoda, H.; Nakajima, M.; Ksouri, R. Formulation, Physicochemical Characterization, and Anti- E. Coli Activity of Food-Grade Nanoemulsions Incorporating Clove, Cinnamon, and Lavender Essential Oils. Food Chem. 2021, 359, 129963. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Liu, J.; Zhao, W.; Huang, H.; Hu, D.; Chen, H.; Zhang, H.; Chen, W.; Gu, Z. Synergistic Effect of Eugenol and Probiotic Lactobacillus Plantarum Zs2058 against Salmonella Infection in C57bl/6 Mice. Nutrients 2020, 12, 1611. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, M.; Zihler Berner, A.; Chervet, N.; Chassard, C.; Lacroix, C. Comparison of the Caco-2, HT-29 and the Mucus-Secreting HT29-MTX Intestinal Cell Models to Investigate Salmonella Adhesion and Invasion. J. Microbiol. Methods 2013, 94, 274–279. [Google Scholar] [CrossRef]
- El Khoury, F.; Corcos, L.; Durand, S.; Simon, B.; Le Jossic-Corcos, C. Acquisition of Anticancer Drug Resistance Is Partially Associated with Cancer Stemness in Human Colon Cancer Cells. Int. J. Oncol. 2016, 49, 2558–2568. [Google Scholar] [CrossRef] [PubMed]
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]



| Run | Experimental Run Number | Parameters | Experimental Response | Fitted Response | ||
|---|---|---|---|---|---|---|
| % Oil Phase [O] | % Substitution Subs | % Surfactant [S] | Z–ave (nm) | Z–ave (nm) | ||
| 1 | 19 | 20.0 | 0 | 1.0 | 366.0 | 394.0 |
| 2 | 7 | 2.5 | 0 | 1.0 | 160.2 | 172.4 |
| 3 | 1 | 2.5 | 100 | 4.0 | 255.1 | 228.4 |
| 4 | 15 | 2.5 | 100 | 1.0 | 144.8 | 166.9 |
| 5 | 14 | 11.3 | 0 | 2.5 | 245.9 | 167.5 |
| 6 | 9 | 2.5 | 50 | 2.5 | 134.9 | 68.3 |
| 7 | 18 | 20.0 | 100 | 1.0 | 697.8 | 640.0 |
| 8 | 3 | 11.3 | 50 | 4.0 | 177.8 | 177.2 |
| 9 | 4 | 2.5 | 0 | 4.0 | 130.0 | 189.1 |
| 10 | 12 | 11.3 | 50 | 1.0 | 226.0 | 221.6 |
| 11 | 5 | 20.0 | 0 | 4.0 | 264.5 | 243.7 |
| 12 | 8 | 20.0 | 50 | 2.5 | 270.6 | 332.1 |
| 13 | 16 | 20.0 | 100 | 4.0 | 545.4 | 534.5 |
| 14 | 11 | 11.3 | 100 | 2.5 | 236.9 | 310.2 |
| 15 | 17 | 11.3 | 50 | 2.5 | 151.0 | 158.7 |
| 16 | 10 | 11.3 | 50 | 2.5 | 153.9 | 158.7 |
| 17 | 13 | 11.3 | 50 | 2.5 | 157.5 | 158.7 |
| 18 | 6 | 11.3 | 50 | 2.5 | 157.4 | 158.7 |
| 19 | 2 | 11.3 | 50 | 2.5 | 163.4 | 158.7 |
| Variable | Z–ave | ||||
|---|---|---|---|---|---|
| Sum of Squares | Df | Mean Square | F–Ratio | p–Value | |
| Main effect | |||||
| A: [O] | 174,029 | 1 | 174,029 | 53.28 | 0 |
| B: Subs | 50,889.2 | 1 | 50,889.2 | 15.58 | 0.0034 |
| C: [S] | 4931.36 | 1 | 4931.36 | 1.51 | 0.2503 |
| Interaction effect | |||||
| AB | 31,626.1 | 1 | 31,626.1 | 9.68 | 0.0125 |
| AC | 13,938.9 | 1 | 13,938.9 | 4.27 | 0.0688 |
| BC | 1000.54 | 1 | 1000.54 | 0.31 | 0.5934 |
| Quadratic effect | |||||
| AA | 4711.4 | 1 | 4711.4 | 1.44 | 0.2604 |
| BB | 17,578.3 | 1 | 17,578.3 | 5.38 | 0.0455 |
| CC | 4531.61 | 1 | 4531.61 | 1.39 | 0.2691 |
| Total error | 29,396.4 | 9 | 3266.27 | ||
| Total (corr.) | 0.16996 | 18 | |||
| R–squared | 0.93 | ||||
| R–squared (adjusted for d.f.) | 0.85 | ||||
| Test | CEO | |
|---|---|---|
| Centrifugation | √ | |
| Freeze–thaw cycle | √ | |
| Heating–cooling cycle | √ | |
| Z–ave (nm) | PdI | |
| Fresh | 93.19 ± 3.92 | 0.1 ± 0.04 |
| 4 °C | 146.56 ± 23.62 | 0.08 ± 0.05 |
| 20 °C | 272.88 ± 32.97 | 0.13 ± 0.07 |
| 37 °C | 673.90 ± 19.07 | 0.22 ± 0.03 |
| IC50 (µg CEO/mL) | TEAC | TPC (mg of GA/mL) | |
|---|---|---|---|
| CEO | 0.78 ± 0.04 a | 97.95 ± 9.91 a | 683.13 ± 77.85 a |
| CEO–NE | 2.43 ± 0.07 b | 31.30 ± 1.7 b | 736.04 ± 13.94 a |
| Microbial Strains | Sample | S. aureus | L. monocytogenes | E. coli | S. typhimurium | Lpb. plantarum | Lb. acidophilus | Lcb. rhamnosus |
|---|---|---|---|---|---|---|---|---|
| Well diffusion agar (mm) | CEO–NE | 10.33 ± 0.58 aA | 10.83 ± 0.76 aA | 17.67 ± 0.58 bB | 11.33 ± 0.29 aA | ND | ND | ND |
| Clove oil | 11.50 ± 0.50 aAB | 11.00 ± 0.50 aA | 12.33 ± 0.58 aB | 11.17 ± 0.29 aA | ND | ND | ND | |
| MIC (mg/mL) | CEO–NE | 0.75 | 0.75 | 0.375 | 0.1875 | 0.1875 | 0.1875 | 0.375 |
| Clove oil | 0.375 | 0.375 | 0.1875 | 0.0937 | 0.0468 | 0.0468 | 0.0468 | |
| MBC (mg/mL) | CEO–NE | 0.75 | 0.75 | 0.375 | 0.375 | 0.75 | 0.75 | 0.75 |
| Clove oil | 0.375 | 0.75 | 0.1875 | 0.1875 | 0.375 | 0.75 | 0.0468 |
| HT–29 | Caco–2 | |
|---|---|---|
| Control | 438.7 ± 71.6 Aa | 538.5 ± 32.5 Cb |
| CEO | 231.0 ± 13.8 Ba | 283.3 ± 9.3 Bb |
| NECEO | 306.6 ± 24.1 Ab | 227.2 ± 5.2 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haro-González, J.N.; Schlienger de Alba, B.N.; Martínez-Velázquez, M.; Castillo-Herrera, G.A.; Espinosa-Andrews, H. Optimization of Clove Oil Nanoemulsions: Evaluation of Antioxidant, Antimicrobial, and Anticancer Properties. Colloids Interfaces 2023, 7, 64. https://doi.org/10.3390/colloids7040064
Haro-González JN, Schlienger de Alba BN, Martínez-Velázquez M, Castillo-Herrera GA, Espinosa-Andrews H. Optimization of Clove Oil Nanoemulsions: Evaluation of Antioxidant, Antimicrobial, and Anticancer Properties. Colloids and Interfaces. 2023; 7(4):64. https://doi.org/10.3390/colloids7040064
Chicago/Turabian StyleHaro-González, José Nabor, Brenda Nathalie Schlienger de Alba, Moisés Martínez-Velázquez, Gustavo Adolfo Castillo-Herrera, and Hugo Espinosa-Andrews. 2023. "Optimization of Clove Oil Nanoemulsions: Evaluation of Antioxidant, Antimicrobial, and Anticancer Properties" Colloids and Interfaces 7, no. 4: 64. https://doi.org/10.3390/colloids7040064