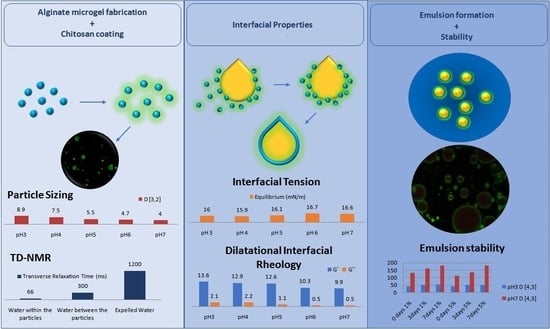
Alginate-Chitosan Microgel Particles, Water–Oil Interfacial Layers, and Emulsion Stabilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Chitosan-Coated Alginate Microgels
2.2.1. Alginate Microgel Preparation
2.2.2. Chitosan Coating
2.3. Particle Sizing of Microgels
2.4. 1H Relaxometry
2.5. Confocal Laser Scanning Microscopy (CLSM)
2.6. Dynamic Interfacial Tension and Dilatational Interfacial Rheology
2.7. Preparation and Characterization of Microgel-Stabilized Emulsion
2.7.1. Emulsion Preparation
2.7.2. Characterization of Emulsion Droplet Size
2.7.3. Emulsion Microstructure
2.7.4. Emulsion Stability Analysis
3. Results
3.1. Particle Characteristics
3.2. Interfacial Properties
3.3. Emulsion Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brugger, B.; Richtering, W. Emulsions Stabilized by Stimuli-Sensitive Poly(N-Isopropylacrylamide)-Co-Methacrylic Acid Polymers: Microgels versus Low Molecular Weight Polymers. Langmuir 2008, 24, 7769–7777. [Google Scholar] [CrossRef]
- Dickinson, E. Microgels—An Alternative Colloidal Ingredient for Stabilization of Food Emulsions. Trends Food Sci. Technol. 2015, 43, 178–188. [Google Scholar] [CrossRef]
- Lyon, L.A.; Fernandez-Nieves, A. The Polymer/Colloid Duality of Microgel Suspensions. Annu. Rev. Phys. Chem. 2012, 63, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Ngai, T.; Auweter, H.; Behrens, S.H. Environmental Responsiveness of Microgel Particles and Particle-Stabilized Emulsions. Macromolecules 2006, 39, 8171–8177. [Google Scholar] [CrossRef]
- Liang, J.; Teng, F.; Chou, T.M.; Libera, M. Measuring Microgel Swell Ratio by Cryo-SEM. Polymer 2017, 116, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, S.; Liu, T.; Rütten, S.; Phan, K.H.; Möller, M.; Richtering, W. Influence of Microgel Architecture and Oil Polarity on Stabilization of Emulsions by Stimuli-Sensitive Core-Shell Poly(N-Isopropylacrylamide-Co-Methacrylic Acid) Microgels: Mickering versus Pickering Behavior? Langmuir 2011, 27, 9801–9806. [Google Scholar] [CrossRef]
- Wu, Y.; Wiese, S.; Balaceanu, A.; Richtering, W.; Pich, A. Behavior of Temperature-Responsive Copolymer Microgels at the Oil/Water Interface. Langmuir 2014, 30, 7660–7669. [Google Scholar] [CrossRef]
- Ishii, T.; Matsumiya, K.; Aoshima, M.; Matsumura, Y. Microgelation Imparts Emulsifying Ability to Surface-Inactive Polysaccharides—Bottom-up vs Top-down Approaches. NPJ Sci. Food 2018, 2, 15. [Google Scholar] [CrossRef]
- Hu, M.; Wu, Y.; Wang, J.; Lu, W.; Gao, Z.; Xu, L.; Cui, S.; Fang, Y.; Nishinari, K. Emulsions Stabilization and Lipid Digestion Profiles of Sodium Alginate Microgels: Effect of the Crosslink Density. Food Biophys. 2021, 16, 346–354. [Google Scholar] [CrossRef]
- Rayner, M. Current Status on Novel Ways for Stabilizing Food Dispersions by Oleosins, Particles and Microgels. Curr. Opin. Food Sci. 2015, 3, 94–109. [Google Scholar] [CrossRef]
- Kwok, M.H.; Sun, G.; Ngai, T. Microgel Particles at Interfaces: Phenomena, Principles, and Opportunities in Food Sciences. Langmuir 2019, 35, 4205–4217. [Google Scholar] [CrossRef]
- Kwok, M.H.; Ngai, T. A Confocal Microscopy Study of Micron-Sized Poly(N-Isopropylacrylamide) Microgel Particles at the Oil-Water Interface and Anisotopic Flattening of Highly Swollen Microgel. J. Colloid Interface Sci. 2016, 461, 409–418. [Google Scholar] [CrossRef]
- Brugger, B.; Vermant, J.; Richtering, W. Interfacial Layers of Stimuli-Responsive Poly-(N-Isopropylacrylamide-Co-Methacrylicacid) (PNIPAM-Co-MAA) Microgels Characterized by Interfacial Rheology and Compression Isotherms. Phys. Chem. Chem. Phys. 2010, 12, 14573–14578. [Google Scholar] [CrossRef]
- Destribats, M.; Lapeyre, V.; Sellier, E.; Leal-Calderon, F.; Schmitt, V.; Ravaine, V. Water-in-Oil Emulsions Stabilized by Water-Dispersible Poly(N-Isopropylacrylamide) Microgels: Understanding Anti-Finkle Behavior. Langmuir 2011, 27, 14096–14107. [Google Scholar] [CrossRef]
- Dickinson, E. Biopolymer-Based Particles as Stabilizing Agents for Emulsions and Foams. Food Hydrocoll. 2017, 68, 219–231. [Google Scholar] [CrossRef]
- Torres, O.; Murray, B.; Sarkar, A. Emulsion Microgel Particles: Novel Encapsulation Strategy for Lipophilic Molecules. Trends Food Sci. Technol. 2016, 55, 98–108. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Physical and Chemical Stability of β-Carotene-Enriched Nanoemulsions: Influence of PH, Ionic Strength, Temperature, and Emulsifier Type. Food Chem. 2012, 132, 1221–1229. [Google Scholar] [CrossRef]
- Destribats, M.; Eyharts, M.; Lapeyre, V.; Sellier, E.; Varga, I.; Ravaine, V.; Schmitt, V. Impact of PNIPAM Microgel Size on Its Ability to Stabilize Pickering Emulsions. Langmuir 2014, 30, 1768–1777. [Google Scholar] [CrossRef]
- De Freitas, R.A.; Nicolai, T.; Chassenieux, C.; Benyahia, L. Stabilization of Water-in-Water Emulsions by Polysaccharide-Coated Protein Particles. Langmuir 2016, 32, 1227–1232. [Google Scholar] [CrossRef]
- Paques, J.P.; Van Der Linden, E.; Van Rijn, C.J.M.; Sagis, L.M.C. Preparation Methods of Alginate Nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171. [Google Scholar] [CrossRef]
- Pravinata, L.; Akhtar, M.; Bentley, P.J.; Mahatnirunkul, T.; Murray, B.S. Preparation of Alginate Microgels in a Simple One Step Process via the Leeds Jet Homogenizer. Food Hydrocoll. 2016, 61, 77–84. [Google Scholar] [CrossRef]
- Burey, P.; Bhandari, B.R.; Howes, T.; Gidley, M.J. Hydrocolloid Gel Particles: Formation, Characterization, and Application. Crit. Rev. Food Sci. Nutr. 2008, 48, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Farjami, T.; Madadlou, A. Fabrication Methods of Biopolymeric Microgels and Microgel-Based Hydrogels. Food Hydrocoll. 2017, 62, 262–272. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Biopolymer-Based Nanoparticles and Microparticles: Fabrication, Characterization, and Application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Murray, B.S. Microgels at Fluid-Fluid Interfaces for Food and Drinks. Adv. Colloid Interface Sci. 2019, 271, 101990. [Google Scholar] [CrossRef]
- Shewan, H.M.; Stokes, J.R. Review of Techniques to Manufacture Micro-Hydrogel Particles for the Food Industry and Their Applications. J. Food Eng. 2013, 119, 781–792. [Google Scholar] [CrossRef]
- Bassani, A.; Montes, S.; Jubete, E.; Palenzuela, J.; Sanjuán, A.P.; Spigno, G. Incorporation of Waste Orange Peels Extracts into PLA Films. Chem. Eng. Trans. 2019, 74, 1063–1068. [Google Scholar] [CrossRef]
- Kavanagh, G.M.; Ross-Murphy, S.B. Rheological characterisation of polymer gels. Prog. Polym. Sci. 1998, 23, 533–562. [Google Scholar] [CrossRef]
- Saavedra Isusi, G.I.; Karbstein, H.P.; van der Schaaf, U.S. Microgel Particle Formation: Influence of Mechanical Properties of Pectin-Based Gels on Microgel Particle Size Distribution. Food Hydrocoll. 2019, 94, 105–113. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, Q.; Liu, Y.C.; Yang, X.Q.; Wang, J.M.; Yin, S.W.; Qi, J.R. Preparation of Soy Protein-Based Microgel Particles Using a Hydrogel Homogenizing Strategy and Their Interfacial Properties. Food Hydrocoll. 2016, 58, 324–334. [Google Scholar] [CrossRef]
- Jiao, B.; Shi, A.; Wang, Q.; Binks, B.P. High-Internal-Phase Pickering Emulsions Stabilized Solely by Peanut-Protein-Isolate Microgel Particles with Multiple Potential Applications. Angew. Chem. Int. Ed. 2018, 57, 9274–9278. [Google Scholar] [CrossRef]
- Matsumiya, K.; Murray, B.S. Soybean Protein Isolate Gel Particles as Foaming and Emulsifying Agents. Food Hydrocoll. 2016, 60, 206–215. [Google Scholar] [CrossRef]
- Sarkar, A.; Murray, B.; Holmes, M.; Ettelaie, R.; Abdalla, A.; Yang, X. In Vitro Digestion of Pickering Emulsions Stabilized by Soft Whey Protein Microgel Particles: Influence of Thermal Treatment. Soft Matter 2016, 12, 3558–3569. [Google Scholar] [CrossRef]
- Zhang, S.; Holmes, M.; Ettelaie, R.; Sarkar, A. Pea Protein Microgel Particles as Pickering Stabilisers of Oil-in-Water Emulsions: Responsiveness to PH and Ionic Strength. Food Hydrocoll. 2020, 102, 105583. [Google Scholar] [CrossRef]
- Mumper, R.J.; Hoffman, A.S.; Puolakkainenb, P.A.; Bouchardb, L.S.; Gombotzb, W.R. Calcium-Alginate Beads for the Oral Delivery of Transforming Growth Factor-PI (TGF-P1): Stabilization of TGF-PI by the Addition of Polyacrylic Acid within Acid-Treated Beads. J. Control. Release 1994, 30, 241–251. [Google Scholar] [CrossRef]
- Segi, N.; Yotsuyanagi, T.; Ikeda, K. Interaction of Calcium-Induced Alginate Gel Beads with Propranolol. Chem. Pharm. Bull. 1989, 37, 3092–3095. [Google Scholar] [CrossRef] [Green Version]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Klinkesorn, U. The Role of Chitosan in Emulsion Formation and Stabilization. Food Rev. Int. 2013, 29, 371–393. [Google Scholar] [CrossRef]
- Li, X.; Xia, W. Effects of Concentration, Degree of Deacetylation and Molecular Weight on Emulsifying Properties of Chitosan. Int. J. Biol. Macromol. 2011, 48, 768–772. [Google Scholar] [CrossRef]
- Payet, L.; Terentjev, E.M. Emulsification and Stabilization Mechanisms of O/W Emulsio ns in the Presence of Chitosan. Langmuir 2008, 24, 12247–12252. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Strand, S.P.; Vårum, K.M.; Draget, K.I.; Nordgård, C.T. Chitosan: Gels and Interfacial Properties. Polymers 2015, 7, 552–579. [Google Scholar] [CrossRef] [Green Version]
- Gåserød, O.; Jolliffe, I.G.; Hampson, F.C.; Dettmar, P.W.; Skjak-Brzk, G. The Enhancement of the Bioadhesive Properties of Calcium Alginate Gel Beads by Coating with Chitosan. Int. J. Pharm. 1998, 175, 237–246. [Google Scholar] [CrossRef]
- Imeson, A. (Ed.) Food Stabilisers, Thickeners, and Gelling Agents; Blackwell Pub: Oxford, UK, 2010; ISBN 9781405132671. [Google Scholar] [CrossRef]
- Gombotz, W.R.; Fong Wee, S. Protein Release from Alginate Matrices. Adv. Drug Deliv. Rev. 1998, 31, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. The Influence of Coating Materials on Some Properties of Alginate Beads and Survivability of Microencapsulated Probiotic Bacteria. Int. Dairy J. 2004, 14, 737–743. [Google Scholar] [CrossRef]
- Alexakis, T.; Boadu, D.K.; Quong, D.; Groboillot, A.; O’neill, I.; Poncelet, D.; Neufeld, R.J. Microencapsulation of DNA Within Aiginate Microspheres and Crosslinked Chitosan Membranes for In Vivo Application. Appl. Biochem. Biotechnol. 1995, 50, 93–106. [Google Scholar] [CrossRef]
- Ribeiro, A.J.; Neufeld, R.J.; Arnaud, P.; Chaumeil, J.C. Microencapsulation of Lipophilic Drugs in Chitosan-Coated Alginate Microspheres. Int. J. Pharm. 1999, 187, 115–123. [Google Scholar] [CrossRef]
- Taqieddin, E.; Amiji, M. Enzyme Immobilization in Novel Alginate-Chitosan Core-Shell Microcapsules. Biomaterials 2004, 25, 1937–1945. [Google Scholar] [CrossRef]
- Ribeiro, A.J.; Silva, C.; Ferreira, D.; Veiga, F. Chitosan-Reinforced Alginate Microspheres Obtained through the Emulsification/Internal Gelation Technique. Eur. J. Pharm. Sci. 2005, 25, 31–40. [Google Scholar] [CrossRef] [Green Version]
- You, J.O.; Liu, Y.C.; Peng, C.A. Efficient Gene Transfection Using Chitosan-Alginate Core-Shell Nanoparticles. Int. J. Nanomed. 2006, 1, 173–180. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, W.; Lv, P.; Wang, L.; Ma, G. Preparation and Evaluation of Alginate-Chitosan Microspheres for Oral Delivery of Insulin. Eur. J. Pharm. Biopharm. 2011, 77, 11–19. [Google Scholar] [CrossRef]
- Conti, B.; Colzani, B.; Papetti, A.; Mascherpa, D.; Dorati, R.; Genta, I.; Pruzzo, C.; Signoretto, C.; Zaura, E.; Lingström, P.; et al. Adhesive Microbeads for the Targeting Delivery of Anticaries Agents of Vegetable Origin. Food Chem. 2013, 138, 898–904. [Google Scholar] [CrossRef]
- Feng, R.; Wang, L.; Zhou, P.; Luo, Z.; Li, X.; Gao, L. Development of the PH Responsive Chitosan-Alginate Based Microgel for Encapsulation of Jughans Regia L. Polyphenols under Simulated Gastrointestinal Digestion in Vitro. Carbohydr. Polym. 2020, 250, 116917. [Google Scholar] [CrossRef]
- Seth, A.; Lafargue, D.; Poirier, C.; Péan, J.M.; Ménager, C. Performance of Magnetic Chitosan-Alginate Core-Shell Beads for Increasing the Bioavailability of a Low Permeable Drug. Eur. J. Pharm. Biopharm. 2014, 88, 374–381. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, J.; Zhang, Z.; Chen, W.; Hu, Q.; Wang, Y. Convenient One-Step Approach Based on Stimuli-Responsive Sol-Gel Transition Properties to Directly Build Chitosan-Alginate Core-Shell Beads. Food Hydrocoll. 2019, 87, 253–259. [Google Scholar] [CrossRef]
- Dickinson, E. Flocculation of Protein-Stabilized Oil-in-Water Emulsions. Colloids Surf. B 2010, 81, 130–140. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.A. Emulsions Stabilized with Solid Nanoparticles: Pickering Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Schroën, K. Pickering Emulsions for Food Applications: Background, Trends, and Challenges. Annu. Rev. Food Sci. Technol. 2015, 6, 263–297. [Google Scholar] [CrossRef]
- Richtering, W. Responsive Emulsions Stabilized by Stimuli-Sensitive Microgels: Emulsions with Special Non-Pickering Properties. Langmuir 2012, 28, 17218–17229. [Google Scholar] [CrossRef]
- Zhou, G.; Lu, Y.; Zhang, H.; Chen, Y.; Yu, Y.; Gao, J.; Sun, D.; Zhang, G.; Zou, H.; Zhong, Y. A Novel Pulsed Drug-Delivery System: Polyelectrolyte Layer-by-Layer Coating of Chitosan-Alginate Microgels. Int. J. Nanomed. 2013, 8, 877–887. [Google Scholar] [CrossRef] [Green Version]
- Duffy, C.; O’Sullivan, M.; Jacquier, J.C. Preparation of Novel Chitosan Iron Microgel Beads for Fortification Applications. Food Hydrocoll. 2018, 84, 608–615. [Google Scholar] [CrossRef]
- Yeung, T.W.; Üçok, E.F.; Tiani, K.A.; McClements, D.J.; Sela, D.A. Microencapsulation in Alginate and Chitosan Microgels to Enhance Viability of Bifidobacterium Longum for Oral Delivery. Front. Microbiol. 2016, 7, 494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matricardi, P.; Di Meo, C.; Coviello, T.; Alhaique, F. Recent Advances and Perspectives on Coated Alginate Microspheres for Modified Drug Delivery. Expert Opin. Drug Deliv. 2008, 5, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Nan, F.; Wu, J.; Qi, F.; Liu, Y.; Ngai, T.; Ma, G. Uniform Chitosan-Coated Alginate Particles as Emulsifiers for Preparation of Stable Pickering Emulsions with Stimulus Dependence. Colloids Surf. A Physicochem. Eng. Asp. 2014, 456, 246–252. [Google Scholar] [CrossRef]
- Provencher, S.W. A constrained regularization method for inverting data represented by linear algebraic or integral equations. Comput. Phys. Commun. 1982, 27, 213–227. [Google Scholar] [CrossRef]
- Huang, M.; Khor, E.; Lim, L.Y. Uptake and Cytotoxicity of Chitosan Molecules and Nanoparticles: Effects of Molecular Weight and Degree of Deacetylation. Pharm. Res. 2004, 21, 344–353. [Google Scholar] [CrossRef]
- Fernández-Nieves, A.; Fernández-Barbero, A.; Vincent, B.; De Las Nieves, F.J. Charge Controlled Swelling of Microgel Particles. Macromolecules 2000, 33, 2114–2118. [Google Scholar] [CrossRef]
- Forshult, S.E. Quantitative Analysis with Pulsed NMR and the CONTIN Computer Program. 2004. Available online: http://urn.kb.se/resolve?urn=urn:nbn:se:kau:diva-2588 (accessed on 20 December 2022).
- Peters, J.P.C.M.; Vergeldt, F.J.; Van As, H.; Luyten, H.; Boom, R.M.; van der Goot, A.J. Time Domain Nuclear Magnetic Resonance as a Method to Determine and Characterize the Water-Binding Capacity of Whey Protein Microparticles. Food Hydrocoll. 2016, 54, 170–178. [Google Scholar] [CrossRef]
- Averardi, A.; Cola, C.; Zeltmann, S.E.; Gupta, N. Effect of Particle Size Distribution on the Packing of Powder Beds: A Critical Discussion Relevant to Additive Manufacturing. Mater. Today 2020, 24, 100964. [Google Scholar] [CrossRef]
- Dopierala, K.; Javadi, A.; Krägel, J.; Schano, K.H.; Kalogianni, E.P.; Leser, M.E.; Miller, R. Dynamic Interfacial Tensions of Dietary Oils. Colloids Surf. A Physicochem. Eng. Asp. 2011, 382, 261–265. [Google Scholar] [CrossRef]
- Li, Z.; Richtering, W.; Ngai, T. Poly(N-Isopropylacrylamide) Microgels at the Oil-Water Interface: Temperature Effect. Soft Matter 2014, 10, 6182–6191. [Google Scholar] [CrossRef]
- Monteux, C.; Marliére, C.; Paris, P.; Pantoustier, N.; Sanson, N.; Perrin, P. Poly(N-Isopropylacrylamide) Microgels at the Oil-Water Interface: Interfacial Properties as a Function of Temperature. Langmuir 2010, 26, 13839–13846. [Google Scholar] [CrossRef]
- Deshmukh, O.S.; Van Den Ende, D.; Stuart, M.C.; Mugele, F.; Duits, M.H.G. Hard and Soft Colloids at Fluid Interfaces: Adsorption, Interactions, Assembly & Rheology. Adv. Colloid Interface Sci. 2015, 222, 215–227. [Google Scholar] [CrossRef]
- Fernandez-Rodriguez, M.A.; Martín-Molina, A.; Maldonado-Valderrama, J. Microgels at Interfaces, from Mickering Emulsions to Flat Interfaces and Back. Adv. Colloid Interface Sci. 2021, 288, 102350. [Google Scholar] [CrossRef]
- Marinova, K.G.; Alargova, R.G.; Denkov, N.D.; Velev, O.D.; Petsev, D.N.; Ivanov, I.B.; Borwankar, R.P. Charging of Oil-Water Interfaces Due to Spontaneous Adsorption of Hydroxyl Ions. Langmuir 1996, 12, 2045–2051. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charisis, A.; Kalogianni, E.P. Alginate-Chitosan Microgel Particles, Water–Oil Interfacial Layers, and Emulsion Stabilization. Colloids Interfaces 2023, 7, 48. https://doi.org/10.3390/colloids7020048
Charisis A, Kalogianni EP. Alginate-Chitosan Microgel Particles, Water–Oil Interfacial Layers, and Emulsion Stabilization. Colloids and Interfaces. 2023; 7(2):48. https://doi.org/10.3390/colloids7020048
Chicago/Turabian StyleCharisis, Aggelos, and Eleni P. Kalogianni. 2023. "Alginate-Chitosan Microgel Particles, Water–Oil Interfacial Layers, and Emulsion Stabilization" Colloids and Interfaces 7, no. 2: 48. https://doi.org/10.3390/colloids7020048