Green Synthesis of Antibacterial Silver Nanocolloids with Agroindustrial Waste Extracts, Assisted by LED Light
Abstract
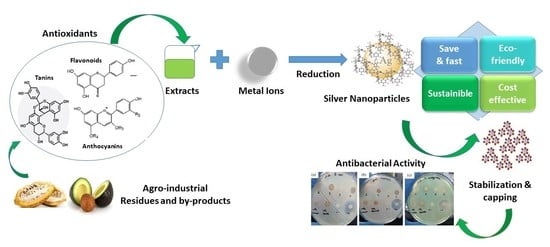
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extract Preparation and Soxhlet Extraction
2.3. Synthesis of AgNPs, Assisted by Light
2.4. Analytical Methods
2.5. Determination of Antioxidant Activity by Ferric Reducing Antioxidant Power (FRAP) Assay
2.6. Determination of Total Polyphenols
2.7. Evaluation of Antibacterial Activity in Vitro
3. Results and Discussion
3.1. Nanoparticle Synthesis
3.2. Determination of Antioxidant Activity and Polyphenol Content of Extracts from Agroindustrial Residues
3.3. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Girotto, F.; Alibardi, L.; Cossu, R. Food waste generation and industrial uses: A review. Waste Manag. 2015, 45, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Orejuela-Escobar, L.M.; Landázuri, A.C.; Goodell, B. Second generation biorefining in Ecuador: Circular bioeconomy, zero waste technology, environment and sustainable development: The nexus. J. Bioresour. Bioprod. 2021, 6, 83–107. [Google Scholar] [CrossRef]
- Ponce, S.; Mena-Campoverde, C.; Proaño, J.S.; Álvarez-Barreto, J.F.; Aguirre, F.; Quintana, D.T.; Sanchez Prieto, J.S.; Streitwieser, D.A. Proposal of a regulatory framework for bioenergy implementation in a unified agricultural code for Ecuador. Biofuels Bioprod. Biorefining 2022, 16, 1116–1129. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Verma, R.; Bhardwaj, P.; Sharma, S.; Kumar, D. Fruit and Vegetable Peels: Utilization of High Value Horticultural Waste in Novel Industrial Applications. Molecules 2020, 25, 2812. [Google Scholar] [CrossRef]
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Fruit peel waste: Characterization and its potential uses. Curr. Sci. 2017, 113, 444–454. [Google Scholar] [CrossRef]
- Mora-Sandí, A.; Ramírez-González, A.; Castillo-Henríquez, L.; Lopretti-Correa, M.; Vega-Baudrit, J.R. Persea Americana Agro-Industrial Waste Biorefinery for Sustainable High-Value-Added Products. Polymers 2021, 13, 1727. [Google Scholar] [CrossRef]
- Riera, M.A.; Maldonado, S.; Palma, R. Residuos agroindustriales generados en ecuador para la elaboración de bioplásticos. Rev. Ing. Ind. 2019, 17, 227–246. [Google Scholar] [CrossRef]
- Dahiya, S.; Kumar, A.N.; Shanthi Sravan, J.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Food waste biorefinery: Sustainable strategy for circular bioeconomy. Bioresour. Technol. 2018, 248, 2–12. [Google Scholar] [CrossRef]
- Aboyewa, J.A.; Sibuyi, N.R.S.; Meyer, M.; Oguntibeju, O.O. Green Synthesis of Metallic Nanoparticles Using Some Selected Medicinal Plants from Southern Africa and Their Biological Applications. Plants 2021, 10, 1929. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Silva, L.P.; Reis, I.G.; Bonatto, C.C. Green Synthesis of Metal Nanoparticles by Plants: Current Trends and Challenges. In Green Processes for Nanotechnology: From Inorganic to Bioinspired Nanomaterials; Basiuk, V.A., Basiuk, E.V., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 259–275. ISBN 978-3-319-15461-9. [Google Scholar]
- Gherasim, O.; Puiu, R.A.; Bîrcă, A.C.; Burdușel, A.-C.; Grumezescu, A.M. An Updated Review on Silver Nanoparticles in Biomedicine. Nanomaterials 2020, 10, 2318. [Google Scholar] [CrossRef] [PubMed]
- Bapat, R.A.; Chaubal, T.V.; Joshi, C.P.; Bapat, P.R.; Choudhury, H.; Pandey, M.; Gorain, B.; Kesharwani, P. An overview of application of silver nanoparticles for biomaterials in dentistry. Mater. Sci. Eng. C 2018, 91, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Moreno, K.; Gonzalez, E.B.; Girón-Vazquez, N.; Chávez-Santoscoy, R.A.; Mota-Morales, J.D.; Perez-Mozqueda, L.L.; Garcia-Garcia, M.R.; Pestryakov, A.; Bogdanchikova, N. Comparison of cytotoxicity and genotoxicity effects of silver nanoparticles on human cervix and breast cancer cell lines. Hum. Exp. Toxicol. 2016, 36, 931–948. [Google Scholar] [CrossRef] [PubMed]
- Narayanamma, A.; Rani, A.; Raju, M.E. Natural synthesis of silver nanoparticles by banana peel extract and as an antibacterial agent. J. Polym. Text. Eng. 2016, 3, 17–25. [Google Scholar]
- Thatikayala, D.; Jayarambabu, N.; Banothu, V.; Ballipalli, C.B.; Park, J.; Rao, K.V. Biogenic synthesis of silver nanoparticles mediated by Theobroma cacao extract: Enhanced antibacterial and photocatalytic activities. J. Mater. Sci. Mater. Electron. 2019, 30, 17303–17313. [Google Scholar] [CrossRef]
- Girón-Vázquez, N.G.; Gómez-Gutiérrez, C.M.; Soto-Robles, C.A.; Nava, O.; Lugo-Medina, E.; Castrejón-Sánchez, V.H.; Vilchis-Nestor, A.R.; Luque, P.A. Study of the effect of Persea americana seed in the green synthesis of silver nanoparticles and their antimicrobial properties. Results Phys. 2019, 13, 102142. [Google Scholar] [CrossRef]
- Srikar, S.K.; Giri, D.D.; Pal, D.B.; Mishra, P.K.; Upadhyay, S.N. Light induced green synthesis of silver nanoparticles using aqueous extract of Prunus amygdalus. Green Sustain. Chem. 2016, 6, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Madhu, G.; Kumar, A.S.; Nair, S.K. Sunlight-induced honey-mediated green synthesis of silver nanoparticles. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019; Volume 2162, p. 20101. [Google Scholar]
- Siewert, B.; Stuppner, H. The photoactivity of natural products—An overlooked potential of phytomedicines? Phytomedicine 2019, 60, 152985. [Google Scholar] [CrossRef]
- Kumar, B.; Angulo, Y.; Smita, K.; Cumbal, L.; Debut, A. Capuli cherry-mediated green synthesis of silver nanoparticles under white solar and blue LED light. Particuology 2016, 24, 123–128. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Khlebtsov, N.G. On the measurement of gold nanoparticle sizes by the dynamic light scattering method. Colloid J. 2011, 73, 118–127. [Google Scholar] [CrossRef]
- Chouhan, S.; Guleria, S. Green synthesis of AgNPs using Cannabis sativa leaf extract: Characterization, antibacterial, anti-yeast and α-amylase inhibitory activity. Mater. Sci. Energy Technol. 2020, 3, 536–544. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green synthesis of silver nanoparticles using Andean blackberry fruit extract. Saudi J. Biol. Sci. 2017, 24, 45–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.; Pandit, S.; Garnæs, J.; Tunjic, S.; Mokkapati, V.R.S.S.; Sultan, A.; Thygesen, A.; Mackevica, A.; Mateiu, R.V.; Daugaard, A.E.; et al. Green synthesis of gold and silver nanoparticles from Cannabis sativa (Industrial hemp) and their capacity for biofilm inhibition. Int. J. Nanomed. 2018, 13, 3571–3591. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Smita, K.; Cumbal, L.; Camacho, J.; Hernández-gallegos, E.; Chávez-lópez, M.D.G.; Grijalva, M.; Andrade, K. One pot phytosynthesis of gold nanoparticles using Genipa americana fruit extract and its biological applications. Mater. Sci. Eng. C 2016, 62, 725–731. [Google Scholar] [CrossRef]
- Setyawan, H.Y.; Sukardi, S.; Puriwangi, C.A. Phytochemicals properties of avocado seed: A review. IOP Conf. Ser. Earth Environ. Sci. 2021, 733, 012090. [Google Scholar] [CrossRef]
- Segovia, F.J.; Hidalgo, G.I.; Villasante, J.; Ramis, X.; Almajano, M.P. Avocado seed: A comparative study of antioxidant content and capacity in protecting oil models from oxidation. Molecules 2018, 23, 2421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diniardi, E.M.; Argo, B.D.; Wibisono, Y. Antibacterial activity of cocoa pod husk phenolic extract against Escherichia coli for food processing. IOP Conf. Ser. Earth Environ. Sci. 2020, 475, 012006. [Google Scholar] [CrossRef]
- Salayová, A.; Bedlovičová, Z.; Daneu, N.; Baláž, M.; Lukáčová Bujňáková, Z.; Balážová, L.; Tkáčiková, L. Green synthesis of silver nanoparticles with antibacterial activity using various medicinal plant extracts: Morphology and antibacterial efficacy. Nanomaterials 2021, 11, 1005. [Google Scholar] [CrossRef]
- Jalab, J.; Abdelwahed, W.; Kitaz, A.; Al-Kayali, R. Green synthesis of silver nanoparticles using aqueous extract of Acacia cyanophylla and its antibacterial activity. Heliyon 2021, 7, e08033. [Google Scholar] [CrossRef]
- Lateef, A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A.; Akinboro, A.; Oladipo, I.C.; Azeez, L.; Ojo, S.A.; Gueguim-Kana, E.B.; Beukes, L.S. Cocoa pod husk extract-mediated biosynthesis of silver nanoparticles: Its antimicrobial, antioxidant and larvicidal activities. J. Nanostruct. Chem. 2016, 6, 159–169. [Google Scholar] [CrossRef]





| Sample | Antioxidant Activity | Concentration of Total Polyphenols |
|---|---|---|
| Aqueous extracts (10 mg/mL) | µmol Trolox equivalents/mL extract | mg GAE/g extract |
| Avocado seeds | 1323.72 ± 16.78 | 1.54 ± 0.088 |
| Cocoa pod husks | 836.50 ± 14.46 | 0.948 ± 0.059 |
| Sample | Staphylococcus aureus | Escherichia coli | Pseudomonas aeruginosa | |
|---|---|---|---|---|
| Inhibition Zone mm (SD) | Inhibition Zone mm (SD) | Inhibition Zone mm (SD) | ||
| 1 | AS extract | 0.0 | 0.0 | 0.0 |
| 2 | AS-NPs | 0.0 | 10.51 ± 0.097 | 0.0 |
| 3 | CPH extract | 0.0 | 0.0 | 0.0 |
| 4 | CPH-NPs | 11.51 ± 0.097 | 13.48 ± 0.025 | 0.0 |
| Negative control | Distilled water | 0.0 | 0.0 | 0.0 |
| Positive control | Gentamicin Discs 10 µg | 21.10 ± 0.015 | 17.48 ± 0.029 | 24.50 ± 0.045 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cañadas, A.; Gualle, A.; Vizuete, K.; Debut, A.; Rojas-Silva, P.; Ponce, S.; Orejuela-Escobar, L.M. Green Synthesis of Antibacterial Silver Nanocolloids with Agroindustrial Waste Extracts, Assisted by LED Light. Colloids Interfaces 2022, 6, 74. https://doi.org/10.3390/colloids6040074
Cañadas A, Gualle A, Vizuete K, Debut A, Rojas-Silva P, Ponce S, Orejuela-Escobar LM. Green Synthesis of Antibacterial Silver Nanocolloids with Agroindustrial Waste Extracts, Assisted by LED Light. Colloids and Interfaces. 2022; 6(4):74. https://doi.org/10.3390/colloids6040074
Chicago/Turabian StyleCañadas, Ambar, Arleth Gualle, Karla Vizuete, Alexis Debut, Patricio Rojas-Silva, Sebastian Ponce, and Lourdes M. Orejuela-Escobar. 2022. "Green Synthesis of Antibacterial Silver Nanocolloids with Agroindustrial Waste Extracts, Assisted by LED Light" Colloids and Interfaces 6, no. 4: 74. https://doi.org/10.3390/colloids6040074