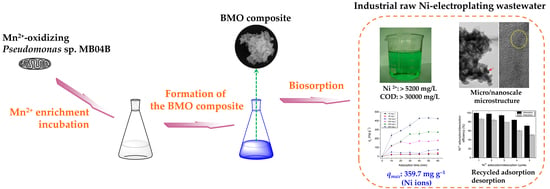
Efficient and Rapid Removal of Nickel Ions from Electroplating Wastewater Using Micro-/Nanostructured Biogenic Manganese Oxide Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Manganese Oxidation Activity Determination
2.3. Preparation of the BMO Composite
2.4. Characterization of the BMO
2.5. Adsorption Experiments
2.6. Kinetics and Isotherm Analysis
2.7. FTIR Spectroscopy
2.8. Data Analysis
3. Results and Discussion
3.1. Characterization of the BMO Aggregate Composite Formed by Mn2+-Oxidizing Pseudomonas sp. MB04B
3.2. Ni2+ Removal Capacity of the Composite
3.3. Adsorption Kinetics
3.4. Isotherm Equation Fitting
3.5. Characterization of Removal Using FTIR and XRD Assays
3.6. Ni2+ Adsorption/Desorption Cycles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Dai, M.; Wu, Y.; Fu, H.; Hou, X.; Peng, C.; Luo, H. Resource utilization of electroplating wastewater: Obstacles and solutions. Environ. Sci. Water Res. Technol. 2022, 8, 484–509. [Google Scholar] [CrossRef]
- Rajoria, S.; Vashishtha, M.; Sangal, V.K. Treatment of electroplating industry wastewater: A review on the various techniques. Environ. Sci. Pollut. Res. 2022, 29, 72196–72246. [Google Scholar] [CrossRef]
- Huang, Z.; Hwang, J.; Huang, C.; Shi, Y. Electroplating Wastewater Treatment in China. Miner. Met. Mater. Ser. 2021, 4, 225–232. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, D.; Wang, W.; Zhong, J.; Feng, K.; Wu, Z.; Du, B.; He, J.; Li, Z.; He, L.; et al. Grave-to-cradle upcycling of Ni from electroplating wastewater to photothermal CO2 catalysis. Nat. Commun. 2022, 13, 5305. [Google Scholar] [CrossRef]
- Noman, E.; Al-Gheethi, A.; Saphira Radin Mohamed, R.M.; Al-Sahari, M.; Hossain, M.S.; Vo, D.-V.N.; Naushad, M. Sustainable approaches for nickel removal from wastewater using bacterial biomass and nanocomposite adsorbents: A review. Chemosphere 2022, 291, 132862. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, Y.; Long, J.; Jiang, D.; Liu, J.; Li, S.; Qi, J.; Zhang, P.; Wang, J.; Gong, J.; et al. Simultaneous removal of thallium and chloride from a highly saline industrial wastewater using modified anion exchange resins. J. Hazard. Mater. 2017, 333, 179–185. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Sparks, D.L. Effect of iron(II) on arsenic sequestration by δ-MnO2: Desorption studies using stirred-flow experiments and X-Ray absorption fine structure spectroscopy. Environ. Sci. Technol. 2015, 49, 13360–13368. [Google Scholar] [CrossRef]
- Fu, X.-Z.; Yang, Y.-R.; Liu, T.; Guo, Z.-Y.; Li, C.-X.; Li, H.-Y.; Cui, K.-P.; Li, W.-W. Biological upcycling of nickel and sulfate as electrocatalyst from electroplating wastewater. Water Res. 2024, 250, 121063. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Li, Y.; Li, Y.; Liu, F.; Liu, Y.; Lu, A.; Wang, C.; Ye, H.; Zhuang, Z. The photogeochemical cycle of Mn oxides on the Earth’s surface. Mineral. Mag. 2021, 85, 22–38. [Google Scholar] [CrossRef]
- Zhou, H.; Fu, C. Manganese-oxidizing microbes and biogenic manganese oxides: Characterization, Mn(II) oxidation mechanism and environmental relevance. Rev. Environ. Sci. Biotechnol. 2020, 19, 489–507. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, K.; Qiu, C.; Bi, Y.; Tian, B.; Bi, X. A review of manganese-oxidizing bacteria (MnOB): Applications, future concerns, and challenges. Int. J. Environ. Res. Public Health 2023, 20, 1272. [Google Scholar] [CrossRef]
- Peacock, C.L.; Sherman, D.M. Sorption of Ni by birnessite: Equilibrium controls on Ni in seawater. Chem. Geol. 2007, 238, 94–106. [Google Scholar] [CrossRef]
- Pena, J.; Bargar, J.R.; Sposito, G. Copper sorption by the edge surfaces of synthetic birnessite nanoparticles. Chem. Geol. 2015, 396, 196–207. [Google Scholar] [CrossRef]
- Yang, P.; Post, J.E.; Wang, Q.; Xu, W.; Geiss, R.; McCurdy, P.R.; Zhu, M. Metal adsorption controls stability of layered manganese oxides. Environ. Sci. Technol. 2019, 53, 7453–7462. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Lee, C.S.; Chang, Y.Y.; Chang, Y.S. Hierarchically structured manganese oxide-coated magnetic nanocomposites for the efficient removal of heavy metal ions from aqueous systems. ACS Appl. Mater. Interfaces 2013, 5, 9628–9634. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-J.; Kim, J.; Choi, S.C.; Chang, Y.S. Sorption behavior of heavy metals on poorly crystalline manganese oxides: Roles of water conditions and light. Environ. Sci. Proc. Impacts 2014, 16, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Chen, X.; Xiong, D.; Liao, S.; Wang, G. Removal and recovery of toxic silver ion using deep-sea bacterial generated biogenic manganese oxides. PLoS ONE 2013, 8, e81627. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Xing, Y.; Qin, X.; Li, X.; Liu, S.; Luo, X.; Huang, Q.; Chen, W. A manganese-oxidizing bacterial consortium and its biogenic Mn oxides for dye decolorization and heavy metal adsorption. Chemosphere 2020, 253, 126627. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huangfu, X.; Ma, C.; Liu, Z. Sequestration and oxidation of heavy metals mediated by Mn(II) oxidizing microorganisms in the aquatic environment. Chemosphere 2023, 329, 138594. [Google Scholar] [CrossRef] [PubMed]
- Krumbein, W.E.; Altmann, H.J. A new method for the detection and enumeration of manganese oxidizing and reducing microorganisms. Helgol. Wiss. Meeresunters 1973, 25, 347–356. [Google Scholar] [CrossRef]
- Wei, S.; Wang, W.; Xiao, F. Biological oxidation of manganese mediated by the fungus Neoroussoella solani MnF107. Int. J. Mol. Sci. 2023, 24, 17093. [Google Scholar] [CrossRef]
- Bai, Y.; Su, J.; Wen, Q.; Li, G.; Xue, L.; Huang, T. Removal of tetracycline by denitrifying Mn(II)-oxidizing bacterium Pseudomonas sp. H117 and biomaterials (BMO and MBMO): Efficiency and mechanisms. Bioresour. Technol. 2020, 312, 123565. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Z.; Zhang, Z.; Chen, H.; Liu, J.; Ali, M.; Liu, F.; Li, L. Population structure of manganese-oxidizing bacteria in stratified soils and properties of manganese oxide aggregates under manganese-complex medium enrichment. PLoS ONE 2013, 8, e73778. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, S.J. Li ion adsorption behaviors of Ni-loaded Li-Mn oxide composites. RSC Adv. 2014, 4, 21899–21903. [Google Scholar] [CrossRef]
- Li, Y.; Ye, D.; Liu, W.; Shi, B.; Guo, R.; Pei, H.; Xie, J. A three-dimensional core-shell nanostructured composite of polypyrrole wrapped MnO2/reduced graphene oxide/carbon nanotube for high performance lithium ion batteries. J. Colloid Interface Sci. 2017, 493, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Liu, F.; Feng, X.; Liu, M.; Tan, W.; Qiu, G. Co2+-exchange mechanism of birnessite and its application for the removal of Pb2+ and As(III). J. Hazard. Mater. 2011, 196, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Ginder-Vogel, M.; Parikh, S.J.; Feng, X.H.; Sparks, D.L. Cation effects on the layer structure of biogenic Mn-oxides. Environ. Sci. Technol. 2010, 44, 4465–4471. [Google Scholar] [CrossRef] [PubMed]
- Ferancová, A.; Hattuniemi, M.K.; Sesay, A.M.; Räty, J.P.; Virtanen, V.T. Complexation of Ni(II) by dimethylglyoxime for rapid removal and monitoring of Ni(II) in water. Mine Water Environ. 2017, 36, 273–282. [Google Scholar] [CrossRef]
- Peng, L.; Zeng, Q.; Tie, B.; Lei, M.; Yang, J.; Luo, S.; Song, Z. Manganese Dioxide nanosheet suspension: A novel absorbent for Cadmium(II) contamination in waterbody. J. Colloid Interface Sci. 2015, 456, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Raj, B.G.S.; Asiri, A.M.; Qusti, A.H.; Wu, J.J.; Anandan, S. Sonochemically synthesized MnO2 nanoparticles as electrode material for supercapacitors. Ultrason. Sonochem. 2014, 21, 1933–1938. [Google Scholar] [CrossRef]
- Jiang, R.; Huang, T.; Liu, J.; Zhuang, J.; Yu, A. A novel method to prepare nanostructured manganese dioxide and its electrochemical properties as a supercapacitor electrode. Electrochim. Acta 2009, 54, 3047–3052. [Google Scholar] [CrossRef]
- Wan, S.; Ma, M.; Lv, L.; Qian, L.; Xu, S.; Xue, Y.; Ma, Z. Selective capture of thallium(I) ion from aqueous solutions by amorphous hydrous manganese dioxide. Chem. Eng. J. 2014, 239, 200–206. [Google Scholar] [CrossRef]
- Omid, H.; Oghabian, M.A.; Ahmadi, R.; Shahbazi, N.; Hosseini, H.R.M.; Shanehsazzadeh, S.; Zangeneh, R.N. Synthesizing and staining manganese oxide nanoparticles for cytotoxicity and cellular uptake investigation. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Ljubic, V.; Perendija, J.; Cvetkovic, S.; Rogan, J.; Trivunac, K.; Stojanovic, M.; Popovic, M. Removal of Ni2+ ions from contaminated water by new exopolysaccharide extracted from K. oxytoca J7 as biosorbent. J. Polym. Environ. 2023. [Google Scholar] [CrossRef]
- Zhu, M.; Ginder-Vogel, M.; Sparks, D.L. Ni(II) sorption on biogenic Mn-oxides with varying Mn octahedral layer structure. Environ. Sci. Technol. 2010, 44, 4472–4478. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Gai, L.; Tang, J.; Fu, J.; Wang, Q.; Zeng, E.Y. Reduction of Cr(VI) in simulated groundwater by FeS-coated iron magnetic nanoparticles. Sci. Total Environ. 2017, 595, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Yun, Y.S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, K.; Wang, H.; Lv, B.; Ma, F. Biosorption of copper and nickel ions using Pseudomonas sp. in single and binary metal systems. Desalination Water Treat. 2016, 57, 2799–2808. [Google Scholar] [CrossRef]
- Naskar, A.; Guha, A.K.; Mukherjee, M.; Ray, L. Adsorption of nickel onto Bacillus cereus M116: A mechanistic approach. Sep. Sci. Technol. 2016, 51, 427–438. [Google Scholar] [CrossRef]
- Chang, Y.S.; Au, P.I.; Mubarak, N.M.; Khalid, M.; Jagadish, P.; Walvekar, R.; Abdullah, E.C. Adsorption of Cu(II) and Ni(II) ions from wastewater onto bentonite and bentonite/GO composite. Environ. Sci. Pollut. Res. 2020, 27, 33270–33296. [Google Scholar] [CrossRef] [PubMed]
- Betiha, M.A.; Moustafa, Y.M.; Mansour, A.S.; Rafik, E.; El-Shahat, M.F. Nontoxic polyvinylpyrrolidone-propylmethacrylate-silica nanocomposite for efficient adsorption of lead, copper, and nickel cations from contaminated wastewater. J. Mol. Liq. 2020, 314, 113656. [Google Scholar] [CrossRef]
- Hu, X.; Yan, L.; Wang, Y.; Xu, M. Smart and functional polyelectrolyte complex hydrogel composed of salecan and chitosan lactate as superadsorbent for decontamination of nickel ions. Int. J. Biol. Macromol. 2020, 165, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.; Yu, Y.; Zhang, X.; Tang, M.; Chen, C.; Wang, S.; Min, D. Phosphorylated wood designed as a biosorbent for effectively removing Ni2+ from wastewater. Ind. Crops Prod. 2022, 188, 115727. [Google Scholar] [CrossRef]







| Levels | Factors | |||
|---|---|---|---|---|
| Temperature (°C) Mean | pH Mean | Adsorption Time (min) Mean | Initial Ni2+ Concentration (mg L−1) Mean | |
| R1 | 34.55 | 37.03 | 38.01 | 94.82 |
| R2 | 42.45 | 40.67 | 40.31 | 17.25 |
| R3 | 42.57 | 41.87 | 41.24 | 7.50 |
| Rj a | 8.01 | 4.84 | 3.23 | 87.32 |
| Rank | 2 | 3 | 4 | 1 |
| C0 (mg L−1) | Equation | R2 | k2 (g mg−1 min−1) | qe (mg g−1) |
|---|---|---|---|---|
| 10 | y = 0.03896x + 0.1755 | 0.9955 | 0.008647 | 25.67 |
| 50 | y = 0.021x + 0.38555 | 0.9024 | 0.001144 | 47.62 |
| 100 | y = 0.01075x + 0.21767 | 0.7915 | 0.000531 | 93.02 |
| 200 | y = 0.00347x + 0.06935 | 0.8185 | 0.000174 | 288.18 |
| 400 | y = 0.00215x + 0.04812 | 0.8811 | 0.000096 | 465.12 |
| 600 | y = 0.00112x + 0.07375 | 0.6934 | 0.000017 | 892.86 |
| Removal Materials | Ni Wastewater | Removal Capacity | Removal Rate | Equilibrium Time | References | |||
|---|---|---|---|---|---|---|---|---|
| Name | Mass | Volume | Concentration | pH | ||||
| Pseudomonas sp. biomass | 1.25 g a | 100 mL | 80 mg L −1 | 4.5 | 336.8 mg g−1 b | − | 40 min | [39] |
| K. oxytoca J7 EPS | 20–200 mg | 5 mL | 10 mg L−1 | 7.2 | 269.97 mg g−1 b | − | 60 min | [34] |
| Bacillus cereus M116 biomass | 0.1 g | 50 mL | 25–1100 mg L−1 | 7.0 | 344.80 mg g−1 b | − | 60 min | [40] |
| Bentonite/GO | 50 mg | 100 mL | 100–500 mg L−1 | 6.0 | 402.5 mg g−1 b | − | 60 min | [41] |
| PVP-SiO2 | 10 mg | 50 mL | 10–200 mg L−1 | 5.0 | 46.1 mg g−1 b | − | 30 min | [42] |
| PEC SC1-SC4 | 100 mg | 200 mL | 600 mg L−1 | 7.0 | 411.8 mg g−1 | 34.3% | 240 min | [43] |
| Phosphorylated wood | 50 mg | 50 mL | 20–200 mg L−1 | 6.0 | 130.2 mg g−1 b | − | 6 h | [44] |
| BMO | 40 mg | 100 mL | 10 mg L−1 | 6.0 | 24.2 mg g−1 | 100% | 20 min | This study |
| 600 mg L−1 | 416.2 mg g−1 | 28.6% | 40 min | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, L.; Liu, Y.; Liu, J.; Li, L. Efficient and Rapid Removal of Nickel Ions from Electroplating Wastewater Using Micro-/Nanostructured Biogenic Manganese Oxide Composite. J. Compos. Sci. 2024, 8, 63. https://doi.org/10.3390/jcs8020063
Li J, Li L, Liu Y, Liu J, Li L. Efficient and Rapid Removal of Nickel Ions from Electroplating Wastewater Using Micro-/Nanostructured Biogenic Manganese Oxide Composite. Journal of Composites Science. 2024; 8(2):63. https://doi.org/10.3390/jcs8020063
Chicago/Turabian StyleLi, Jiaoqing, Li Li, Yongxuan Liu, Jin Liu, and Lin Li. 2024. "Efficient and Rapid Removal of Nickel Ions from Electroplating Wastewater Using Micro-/Nanostructured Biogenic Manganese Oxide Composite" Journal of Composites Science 8, no. 2: 63. https://doi.org/10.3390/jcs8020063