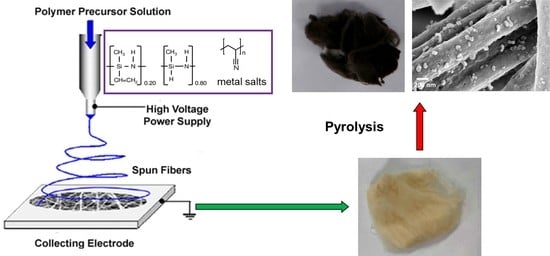
In Situ Formation of Nanoparticles on Carbon Nanofiber Surface Using Ceramic Intercalating Agents
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, Y.; Cao, Y.; Yao, Q.; Chai, O.J.H.; Xie, J. Engineering Noble Metal Nanomaterials for Pollutant Decomposition. Ind. Eng. Chem. Res. 2020, 59, 20561–20581. [Google Scholar] [CrossRef]
- Asensio, J.M.; Bouzouita, D.; van Leeuwen, P.W.N.M.; Chaudret, B. σ-H-H, σ-C-H, and σ-Si-H Bond Activation Catalyzed by Metal Nanoparticles. Chem. Rev. 2020, 120, 1042–1084. [Google Scholar] [CrossRef]
- Narayan, N.; Meiyazhagan, A.; Vajtai, R. Metal nanoparticles as green catalysts. Materials 2019, 12, 3602. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Lu, X.F.; Xia, B.Y.; Lou, X.W. Advanced Electrocatalysts for the Oxygen Reduction Reaction in Energy Conversion Technologies. Joule 2020, 4, 45–68. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef]
- Gerber, I.C.; Serp, P. A Theory/Experience Description of Support Effects in Carbon-Supported Catalysts. Chem. Rev. 2020, 120, 1250–1349. [Google Scholar] [CrossRef]
- Liu, M.; Hof, F.; Moro, M.; Valenti, G.; Paolucci, F.; Pénicaud, A. Carbon supported noble metal nanoparticles as efficient catalysts for electrochemical water splitting. Nanoscale 2020, 12, 20165–20170. [Google Scholar] [CrossRef]
- Zhang, M.; Ding, L.; Zheng, J.; Liu, L.; Alsulami, H.; Kutbi, M.A.; Xu, J. Surface modification of carbon fibers with hydrophilic Fe3O4 nanoparticles for nickel-based multifunctional composites. Appl. Surf. Sci. 2020, 509, 145348. [Google Scholar] [CrossRef]
- Navrotskaya, A.G.; Aleksandrova, D.D.; Krivoshapkina, E.F.; Sillanpää, M.; Krivoshapkin, P.V. Hybrid Materials Based on Carbon Nanotubes and Nanofibers for Environmental Applications. Front. Chem. 2020, 8, 546. [Google Scholar] [CrossRef]
- An, G.H.; Lee, E.H.; Ahn, H.J. Well-dispersed iron nanoparticles exposed within nitrogen-doped mesoporous carbon nanofibers by hydrogen-activation for oxygen-reduction reaction. J. Alloys Compd. 2016, 682, 746–752. [Google Scholar] [CrossRef]
- Wang, W.; Liang, Y.; Kang, Y.; Liu, L.; Xu, Z.; Tian, X.; Mai, W.; Fu, H.; Lv, H.; Teng, K.; et al. Carbon-coated SnO2@carbon nanofibers produced by electrospinning-electrospraying method for anode materials of lithium-ion batteries. Mater. Chem. Phys. 2019, 223, 762–770. [Google Scholar] [CrossRef]
- Abouali, S.; Garakani, M.A.; Zhang, B.; Xu, Z.L.; Heidari, E.K.; Huang, J.Q.; Huang, J.; Kim, J.K. Electrospun Carbon Nanofibers with in Situ Encapsulated Co3O4 Nanoparticles as Electrodes for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 13503–13511. [Google Scholar] [CrossRef]
- Li, T.; Li, S.; Liu, Q.; Yin, J.; Sun, D.; Zhang, M.; Xu, L.; Tang, Y.; Zhang, Y. Immobilization of Ni3Co Nanoparticles into N-Doped Carbon Nanotube/Nanofiber Integrated Hierarchically Branched Architectures toward Efficient Overall Water Splitting. Adv. Sci. 2020, 7, 1902371. [Google Scholar] [CrossRef] [Green Version]
- Bazargan, A.; Esmaeilpour, M.; Keyanpour-Rad, M. Catalytically Graphitized Electrospun Carbon Nanofibers Adorned with Nickel Nanoparticles for Catalysis Applications. J. Nanostructures 2016, 6, 52–57. [Google Scholar] [CrossRef]
- Colombo, P.; Mera, G.; Riedel, R.; Sorarù, G.D. Polymer-derived ceramics: 40 Years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Ren, Z.; Mujib, S.B.; Singh, G. High-temperature properties and applications of Si-based polymer-derived ceramics: A review. Materials 2021, 14, 614. [Google Scholar] [CrossRef]
- Ramlow, H.; Marangoni, C.; Motz, G.; Machado, R.A.F. Statistical optimization of polysilazane-derived ceramic: Electrospinning with and without organic polymer as a spinning aid for manufacturing thinner fibers. Chem. Eng. J. Adv. 2022, 9, 100220. [Google Scholar] [CrossRef]
- Mcenaney, J.M.; Schaak, R.E. Solution synthesis of metal silicide nanoparticles. Inorg. Chem. 2015, 54, 707–709. [Google Scholar] [CrossRef]
- Ryabchuk, P.; Agostini, G.; Pohl, M.M.; Lund, H.; Agapova, A.; Junge, H.; Junge, K.; Beller, M. Intermetallic nickel silicide nanocatalyst—A non-noble metal–based general hydrogenation catalyst. Sci. Adv. 2018, 4, eaat0761. [Google Scholar] [CrossRef] [Green Version]
- Tam, P.L.; Nyborg, L. Sputter deposition and XPS analysis of nickel silicide thin films. Surf. Coatings Technol. 2009, 203, 2886–2890. [Google Scholar] [CrossRef]
- Silva, T.C.d.E.; Mooste, M.; Kibena-Põldsepp, E.; Matisen, L.; Merisalu, M.; Kook, M.; Sammelselg, V.; Tammeveski, K.; Wilhelm, M.; Rezwan, K. Polymer-derived Co/Ni-SiOC(N) ceramic electrocatalysts for oxygen reduction reaction in fuel cells. Catal. Sci. Technol. 2019, 9, 854–866. [Google Scholar] [CrossRef]
- Zhang, C.; Qu, L.; Yuan, W. Effects of Si/C Ratio on the Phase Composition of Si-C-N Powders Synthesized by Carbonitriding. Materials 2020, 13, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markel, I.J.; Glaser, J.; Steinbrück, M.; Seifert, H.J. Experimental and computational analysis of PSZ 10- and PSZ 20-derived Si-C-N ceramics. J. Eur. Ceram. Soc. 2019, 39, 195–204. [Google Scholar] [CrossRef]
- Esconjauregui, S.; Whelan, C.M.; Maex, K. Carbon nanotube catalysis by metal silicide: Resolving inhibition versus growth. Nanotechnology 2007, 18, 015602. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burnstine-Townley, A.A.; Afrin, S.; Li Sip, Y.Y.; Fox, D.; Zhai, L. In Situ Formation of Nanoparticles on Carbon Nanofiber Surface Using Ceramic Intercalating Agents. J. Compos. Sci. 2022, 6, 303. https://doi.org/10.3390/jcs6100303
Burnstine-Townley AA, Afrin S, Li Sip YY, Fox D, Zhai L. In Situ Formation of Nanoparticles on Carbon Nanofiber Surface Using Ceramic Intercalating Agents. Journal of Composites Science. 2022; 6(10):303. https://doi.org/10.3390/jcs6100303
Chicago/Turabian StyleBurnstine-Townley, Alex A., Sajia Afrin, Yuen Yee Li Sip, David Fox, and Lei Zhai. 2022. "In Situ Formation of Nanoparticles on Carbon Nanofiber Surface Using Ceramic Intercalating Agents" Journal of Composites Science 6, no. 10: 303. https://doi.org/10.3390/jcs6100303