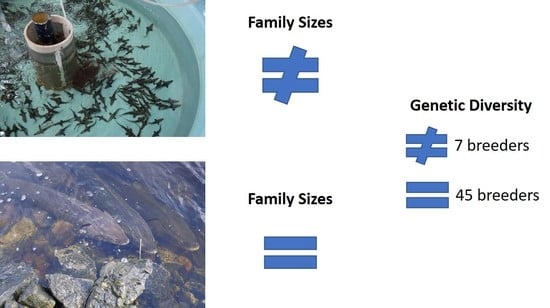
Parentage Analysis Reveals Unequal Family Sizes during Hatchery Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Laboratory Techniques
2.3. Sex Identification
2.4. Parentage Analysis
2.5. Genetic Diversity Comparison between Hatchery- and Wild-Produced Offspring
3. Results
3.1. Sex Identification
3.2. Hatchery-Produced Offspring
3.3. Wild-Produced Larvae
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ford, M.J. Selection in captivity during supportive breeding may reduce fitness in the wild. Conserv. Biol. 2002, 16, 815–825. [Google Scholar] [CrossRef]
- Crossman, J.A.; Scribner, K.T.; Yen, D.T.; Davis, C.A.; Forsythe, P.S.; Baker, E.A. Gamete and larval collection methods and hatchery rearing environments affect levels of genetic diversity in early life stages of lake sturgeon (Acipenser fulvescens). Aquaculture 2011, 310, 312–324. [Google Scholar] [CrossRef]
- Flagg, T.A.; Nash, C.F. A Conceptual Framework for Conservation Hatchery Strategies for Pacific Salmonids; NOAA Technical Memorandum NMFS-NWFSC-38; U.S. Department of Commerce, National Oceanic and Atmospheric Administration: Washington, DC, USA, 1999; 54p.
- Fiumera, A.C.; Porter, B.A.; Looney, G.; Asmussen, M.A.; Avise, J.C. Maximizing offspring production while maintaining genetic diversity in supplemental breeding programs of highly fecund managed species. Conserv. Biol. 2004, 18, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Engström, G.; McMillan, I.; McKay, L.; Quinton, M. Comparison of mating systems in a fish population: A simulation study. J. Anim. Breed. Genet. 1996, 113, 559–566. [Google Scholar] [CrossRef]
- Dupont-Nivet, M.; Vandeputte, M.; Haffray, P.; Chevassus, B. Effect of different mating designs on inbreeding, genetic variance and response to selection when applying individual selection in fish breeding programs. Aquaculture 2006, 252, 161–170. [Google Scholar] [CrossRef]
- Busack, C.; Knudsen, C.M. Using factorial mating designs to increase the effective number of breeders in fish hatcheries. Aquaculture 2007, 273, 24–32. [Google Scholar] [CrossRef]
- Ryman, N.; Laikre, L. Effects of supportive breeding on the genetically effective population size. Conserv. Biol. 1991, 5, 325–329. [Google Scholar] [CrossRef]
- Moyer, G.R.; Blouin, M.S.; Banks, M.A. The influence of family-correlated survival on Nb/N for progeny from integrated multi-and single-generation hatchery stocks of coho salmon (Oncorhynchus kisutch). Can. J. Fish. Aquat. Sci. 2007, 64, 1258–1265. [Google Scholar] [CrossRef]
- Holtgren, J.M.; Ogren, S.A.; Paquet, A.J.; Fajfer, S. Design of a portable streamside rearing facility for lake sturgeon. N. Am. J. Aquacult. 2007, 69, 317–323. [Google Scholar] [CrossRef]
- Campton, D.E. Genetic effects of hatchery fish on wild populations of Pacific salmon and steelhead: What do we really know? Am. Fish. Soc. Symp. 1995, 15, 337–353. [Google Scholar]
- Currens, K.P.; Busack, C.A. A framework for assessing genetic vulnerability. Fisheries 1995, 20, 24–31. [Google Scholar] [CrossRef]
- Welsh, A.B.; Elliott, R.F.; Scribner, K.T.; Quinlan, H.R.; Baker, E.A.; Eggold, B.T.; Holtgren, J.M.; Krueger, C.C.; May, B. Genetic guidelines for the stocking of lake sturgeon (Acipenser fulvescens) in the Great Lakes basin. In Great Lakes Fishery Commission Miscellaneous Publication 2010-01; Great Lakes Fishery Commission: Ann Arbor, MI, USA, 2010; 62p. [Google Scholar]
- May, B.; Krueger, C.C.; Kincaid, H.L. Genetic variation at microsatellite loci in sturgeon: Primer sequence homology in Acipenser and Scaphirhynchus. Can. J. Fish. Aquat. Sci. 1997, 54, 1542–1547. [Google Scholar] [CrossRef]
- King, T.L.; Lubinski, B.A.; Spidle, A.P. Microsatellite DNA variation in Atlantic sturgeon (Acipenser oxyrinchus oxyrinchus) and cross-species amplification in the Acipenseridae. Conserv. Genet. 2001, 2, 103–119. [Google Scholar] [CrossRef]
- McQuown, E.C.; Gall, G.A.E.; May, B. Characterization and inheritance of six microsatellite loci in lake sturgeon. T. Am. Fish. Soc. 2002, 131, 299–307. [Google Scholar] [CrossRef]
- Welsh, A.; Blumberg, M.; May, B. Identification of microsatellite loci in lake sturgeon, Acipenser fulvescens, and their variability in green sturgeon. A. medirostris. Mol. Ecol. Notes 2003, 3, 47–55. [Google Scholar] [CrossRef]
- Estep, K.; VanDeHey, J.; Raabe, J.; Schmalz, P.; Wilfond, D.; Piszczeck, P.; Borkholder, B. Genetic origins and diversity of lake sturgeon in the St. Louis River estuary. J. Great Lakes Res. 2020, 46, 1028–1035. [Google Scholar] [CrossRef]
- Kuhl, H.; Guiguen, Y.; Höhne, C.; Kreuz, E.; Du, K.; Klopp, C.; Lopez-Roques, C.; Yebra-Pimentel, E.S.; Ciorpac, M.; Gessner, J.; et al. A 180 Myr-old female-specific genome region in sturgeon reveals the oldest known vertebrate sex determining system with undifferentiated sex chromosomes. Philos. T. R. Soc. B 2021, 376, 20200089. [Google Scholar] [CrossRef]
- Scribner, K.T.; Kanefsky, J. Molecular sexing of lake sturgeon. J. Great Lakes Res. 2021, 47, 934–936. [Google Scholar] [CrossRef]
- Marshall, T.C.; Slate, J.; Kruuk, L.E.B.; Pemberton, J.M. Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 1998, 7, 639–655. [Google Scholar] [CrossRef] [Green Version]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Santure, A. Parentage and sibship inference from multilocus genotype data under polygamy. Genetics 2009, 181, 1579–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, O.R.; Wang, J. COLONY: A program for parentage and sibship inference from multilocus genotype data. Mol. Ecol. Resour. 2010, 10, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Goudet, J. FSTAT (ver. 2.9.4), a Program to Estimate and Test Population Genetics Parameters. 2003. Available online: http://https://www2.unil.ch/popgen/softwares/fstat.htm (accessed on 21 February 2023).
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin ver. 3.0: An integrated software package for population genetics data analysis. Evol. Bioinform. 2005, 1, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Serbezov, D.; Bernatchez, L.; Olsen, E.M.; Vøllestad, L.A. Mating patterns and determinants of individual reproductive success in brown trout (Salmo trutta) revealed by parentage analysis of an entire stream living population. Mol. Ecol. 2010, 19, 3193–3205. [Google Scholar] [CrossRef]
- Thompson, N.F.; Clemens, B.J.; Ketchum, L.L.; Simpson, P.C.; Reagan, R.E.; Blouin, M.S. Family influence on length at release and size-biased survival post release in hatchery-reared steelhead: A mechanism to explain how genetic adaptation to captivity occurs. Aquaculture 2018, 491, 135–146. [Google Scholar] [CrossRef]
- Blouin, M.S.; Wrey, M.C.; Bollmann, S.R.; Skaar, J.C.; Twibell, R.G.; Fuentes, C. X Offspring of first-generation hatchery steelhead trout (Oncorhynchus mykiss) grow faster in the hatchery than offspring of wild fish, but survive worse in the wild: Possible mechanisms for inadvertent domestication and fitness loss in hatchery salmon. PLoS ONE 2018, 16, e0257407. [Google Scholar] [CrossRef]
- Thompson, N.F.; Blouin, M.S. The effects of high rearing density on the potential for domestication selection in hatchery culture of steelhead (Oncorhynchus mykiss). Can. J. Fish. Aquat. Sci. 2015, 72, 1829–1834. [Google Scholar] [CrossRef]
- Evans, M.L.; Neff, B.D.; Heath, D.D. Behavioural and genetic analyses of mate choice and reproductive success in two Chinook salmon populations. Can. J. Fish. Aquat. Sci. 2013, 70, 263–270. [Google Scholar] [CrossRef]
- Whitcomb, A.C.; Banks, M.A.; O’Malley, K.G. Influence of immune-relevant genes on mate choice and reproductive success in wild-spawning hatchery-reared and wild-born coho salmon (Oncorhynchus kisutch). Can. J. Fish. Aquat. Sci. 2014, 71, 1000–1009. [Google Scholar] [CrossRef]
- Fleming, I.A. Pattern and variability in the breeding system of Atlantic salmon (Salmo salar), with comparisons to other salmonids. Can. J. Fish. Aquat. Sci. 1998, 55, 59–76. [Google Scholar] [CrossRef]
- Ford, M.J.; Murdoch, A.R.; Hughes, M.S.; Seamons, T.R.; LaHood, E.S. Broodstock history strongly influences natural spawning success in hatchery steelhead (Oncorhynchus mykiss). PLoS ONE 2016, 11, e0164801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorstensen, M.; Bates, P.; Lepla, K.; Schreier, A. To breed or not to breed? Maintaining genetic diversity in white sturgeon supplementation programs. Conserv. Genet. 2019, 20, 997–1007. [Google Scholar] [CrossRef]
- Bruch, R.M.; Binkowski, F.P. Spawning behavior of lake sturgeon (Acipenser fulvescens). J. Appl. Ichthyol. 2002, 18, 570–579. [Google Scholar] [CrossRef] [Green Version]
- Allendorf, F.W.; Funk, W.C.; Aitken, S.N.; Byrne, M.; Luikart, G. Conservation and the Genomics of Populations, 3rd ed.; Oxford University Press: Oxford, UK, 2022. [Google Scholar]
- Lande, R. Genetics and demography in biological conservation. Science 1988, 241, 1455–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay-Chmielewski, E.M.; Whelan, G.E. Lake sturgeon rehabilitation strategy. Mich. Dep. Nat. Resour. Fish. Div. Spec. Rep. 1997, 18, 1–51. [Google Scholar]
- Hayes, D.B.; Caroffino, D.C. Michigan’s lake sturgeon rehabilitation strategy. Mich. Dep. Nat. Resour. Fish. Div. Spec. Rep. 2012, 62, 1–26. [Google Scholar]
| Year | Family Group | Male | X2 | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||
| 2017 | 2 | 33 | 5 | 31 | 31 | N/A | 21.44 | 8.53 × 10−5 |
| 2017 | 3 | 21 | 36 | 2 | 19 | N/A | 29.79 | 1.52 × 10−6 |
| 2017 | 4 | 35 | 33 | 2 | 19 | N/A | 31.40 | 6.99 × 10−7 |
| 2017 | 5 | 35 | 38 | 18 | 8 | N/A | 24.52 | 1.95 × 10−5 |
| 2017 | 6 | 23 | 24 | 5 | 10 | N/A | 13.63 | 0.001 |
| 2017 | 7 | 12 | 8 | 51 | 26 | N/A | 46.71 | 4.00 × 10−10 |
| 2017 | 8 | 6 | 30 | 36 | 27 | N/A | 20.64 | 0.0001 |
| 2017 | 9 | 44 | 10 | 24 | 22 | N/A | 23.84 | 2.70 × 10−5 |
| 2017 | 10 | 22 | 19 | 7 | 50 | N/A | 40.53 | 8.22 × 10−9 |
| 2018 | 1 | 34 | 7 | 3 | 28 | 11 | 50.05 | 4.70 × 10−9 |
| N | Nb | Allelic Richness | Observed Heterozygosity | |
|---|---|---|---|---|
| Hatchery–2017 | 862 | 38 (34–42) | 4.33 | 0.548 |
| Hatchery–2018 | 84 | 3 (2–12) | 3.25 | 0.543 |
| Wild-produced | 675 | 127 (110–147) | 4.75 | 0.538 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akers, M.; Quinlan, H.; Johnson, A.; Baker, E.; Welsh, A. Parentage Analysis Reveals Unequal Family Sizes during Hatchery Production. Fishes 2023, 8, 140. https://doi.org/10.3390/fishes8030140
Akers M, Quinlan H, Johnson A, Baker E, Welsh A. Parentage Analysis Reveals Unequal Family Sizes during Hatchery Production. Fishes. 2023; 8(3):140. https://doi.org/10.3390/fishes8030140
Chicago/Turabian StyleAkers, Mary, Henry Quinlan, Andrew Johnson, Edward Baker, and Amy Welsh. 2023. "Parentage Analysis Reveals Unequal Family Sizes during Hatchery Production" Fishes 8, no. 3: 140. https://doi.org/10.3390/fishes8030140