Betholletia excelsa Fruit: Unveiling Toughening Mechanisms and Biomimetic Potential for Advanced Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. C-Ring
2.3. Compression Test
2.4. Shearography
2.5. Microstructural Analysis
2.6. MicroCT
2.7. FEM Analysis
3. Results
3.1. C-Ring Tests
3.2. Compression of Half Mesocarp
3.3. FEM Analysis
4. Discussion
5. Conclusions
- The elastic modulus of C-ring specimens perpendicular to the mesocarp latitudinal section (~4 GPa) is twice the modulus measured parallel to it. This anisotropy is caused by the preferential fiber orientation of layers I and III, the most stressed regions during bending.
- The fracture strength can reach 26.4 ± 3.6 MPa in the compression of C-ring longitudinal specimens. The rough mesocarp surface with folds, dimples, and many stress raisers can easily nucleate cracks during C-ring tests and when decreasing specimens’ mean strength.
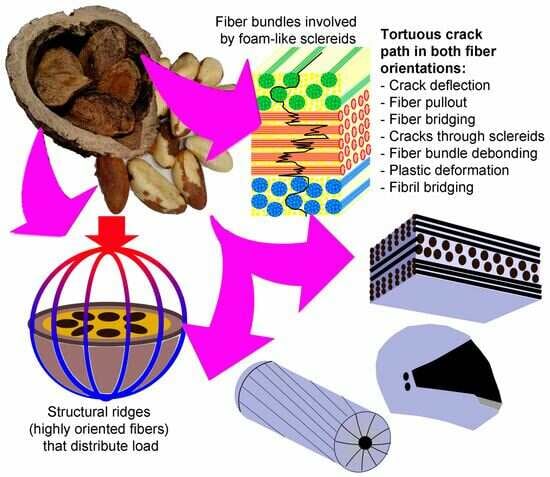
- Fiber bundles orthogonal to the crack path show an intense crack deflection and experienced fiber pullout and fiber bridging before brittle failure, dissipating a great amount of energy. Surprisingly, fiber bundles oriented parallel to the crack path also contibuted to the energy dissipation. Intercellular cracks propagating through fibers and sclereids’ middle lamellae promoted a long and tortuous crack path. After the debonding of fiber bundles, plastic deformation and fibril bridging was observed in the middle lamellae.
- The fiber bundle orientation, the structural ridges, and the specimen geometry together explain the high forces and displacements of longitudinal specimens in the compression test of half mesocarps.
- The FEM analysis indicated that the structural ridges uniformly distributed the stress in the shell only when oriented parallel to the load direction (longitudinal specimens). The fiber and ridge orientations of longitudinal specimens also promoted intense deformation with the formation of a flat top, resulting in the bending of the shell and crack nucleation.
- Crack development in longitudinal specimens was slowed by the breaking of numerous fiber bundles in layer II, positioned orthogonal to the crack path.
- Latitudinal specimens showed stress concentrations in a few ridges below the loaded surface, where the main crack nucleated. Fiber orientation in mesocarp layer II is parallel to the crack path in latitudinal specimens, resulting in the faster crack propagation observed in these specimens.
- The void content did not considerably change after the compression tests, indicating that only voids in the most critical mechanically affected volume developed into growing cracks. However, these structural voids interacted with cracks, mainly attracting, deflecting, and ramifying them.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meyers, M.A.; Chen, P.-Y.; Lin, A.Y.-M.; Seki, Y. Biological Materials: Structure and Mechanical Properties. Prog. Mater. Sci. 2008, 53, 1–206. [Google Scholar] [CrossRef]
- Ashby, M.F.; Medalist, R.F.M. The Mechanical Properties of Cellular Solids. MTA 1983, 14, 1755–1769. [Google Scholar] [CrossRef]
- Barthelat, F.; Yin, Z.; Buehler, M.J. Structure and Mechanics of Interfaces in Biological Materials. Nat. Rev. Mater. 2016, 1, 16007. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Komvopoulos, K. Mechanical Behavior of Bamboo-Like Structures under Transversal Compressive Loading. Biomimetics 2023, 8, 103. [Google Scholar] [CrossRef]
- Compton, B.G.; Lewis, J.A. 3D-Printing of Lightweight Cellular Composites. Adv. Mater. 2014, 26, 5930–5935. [Google Scholar] [CrossRef] [PubMed]
- Staufenberg, G.; Graupner, N.; Müssig, J. Impact and Hardness Optimization of Composite Materials Inspired by the Babassu Nut (Orbignya speciosa). Bioinspir. Biomim. 2015, 10, 056006. [Google Scholar] [CrossRef]
- Li, S.H.; Zeng, Q.Y.; Xiao, Y.L.; Fu, S.Y.; Zhou, B.L. Biomimicry of Bamboo Bast Fiber with Engineering Composite Materials. Mater. Sci. Eng. C 1995, 3, 125–130. [Google Scholar] [CrossRef]
- Tao, J.; Tahmasebi, P.; Kader, M.A.; Feng, D.; Sahimi, M.; Evans, P.D.; Saadatfar, M. Wood Biomimetics: Capturing and Simulating the Mesoscale Complexity of Willow Using Cross-Correlation Reconstruction Algorithm and 3D Printing. Mater. Des. 2023, 228, 111812. [Google Scholar] [CrossRef]
- Fleck, C.; Zaslansky, P.; Currey, J.D.; Meinel, D. Microstructural Features Influencing Failure in Macadamia Nuts. Bioinspired Biomim. Nanobiomater. 2012, 1, 67–75. [Google Scholar] [CrossRef]
- Schuler, P.; Speck, T.; Buhrig-Polaczek, A.; Fleck, C. Structure-Function Relationships in Macadamia Integrifolia Seed Coats-Fundamentals of the Hierarchical Microstructure. PLoS ONE 2014, 9, e0102913. [Google Scholar] [CrossRef]
- Ali, S.A.; Sonego, M.; Salavati, M.; Fleck, C. Influence of Structure and Geometry on the Compressive Deformation Behavior of Macadamia Integrifolia and Bertholletia excelsa Shells: A Validated Finite Element Simulation Study. Adv. Eng. Mater. 2023. [Google Scholar] [CrossRef]
- Antreich, S.J.; Xiao, N.; Huss, J.C.; Horbelt, N.; Eder, M.; Weinkamer, R.; Gierlinger, N. The Puzzle of the Walnut Shell: A Novel Cell Type with Interlocked Packing. Adv. Sci. 2019, 6, 1900644. [Google Scholar] [CrossRef] [PubMed]
- Gludovatz, B.; Walsh, F.; Zimmermann, E.A.; Naleway, S.E.; Ritchie, R.O.; Kruzic, J.J. Journal of the Mechanical Behavior of Biomedical Materials Multiscale Structure and Damage Tolerance of Coconut Shells. J. Mech. Behav. Biomed. Mater. 2017, 76, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Massalter, T.; Bauer, G.; Gallenmuller, F.; Tobias, H.; Poppinga, S.; Schmitt, C.; Seidel, R.; Speck, O.; Thielen, M.; Speck, T. Biomimetic Impact and Puncture-Resistant Materials Based on Fruit Walls and Nut Shells. In Biomimetics: Nature-Based Innovation; Bar-Cohen, Y., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 377–429. ISBN 978-1-4398-3476-3. [Google Scholar]
- Seidel, R. Fruit Wall and Nut Shell as an Inspiration for the Design of Bio-Inspired Impact-Resistant Hierarchically Structured Materials; WIT Press: Billerica, MA, USA, 2013; Volume 8, pp. 172–179. [Google Scholar] [CrossRef]
- Bührig-Polaczek, A.; Fleck, C.; Speck, T.; Schüler, P.; Fischer, S.F.; Caliaro, M.; Thielen, M. Biomimetic Cellular Metals—Using Hierarchical Structuring for Energy Absorption. Bioinspir. Biomim. 2016, 11, 045002. [Google Scholar] [CrossRef] [PubMed]
- Sonego, M.; Fleck, C.; Pessan, L.A. Mesocarp of Brazil Nut (Bertholletia excelsa) as Inspiration for New Impact Resistant Materials. Bioinspir. Biomim. 2019, 14, 056002. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.A.; Prance, G.T. Taxonomy Details. Available online: http://sweetgum.nybg.org/lp/taxon.php?irn=133412 (accessed on 3 August 2016).
- Haugaasen, J.M.T.; Haugaasen, T.; Peres, C.A.; Gribel, R.; Wegge, P. Seed Dispersal of the Brazil Nut Tree (Bertholletia excelsa) by Scatter-Hoarding Rodents in a Central Amazonian Forest. J. Trop. Ecol. 2010, 26, 251–262. [Google Scholar] [CrossRef]
- Sonego, M.; Fleck, C.; Pessan, L.A. Hierarchical Levels of Organization of the Brazil Nut Mesocarp. Sci. Rep. 2020, 10, 6786. [Google Scholar] [CrossRef]
- Mishra, S. Understanding Plant Anatomy; Discovery Publishing House Pvt. Limited: New Delhi, India, 2009; ISBN 9788183564571. [Google Scholar]
- Lawrence, M.; Jiang, Y. Porosity, Pore Size Distribution, Micro-Structure. In Bio-Aggregates Based Building Materials; Amziane, S., Collet, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 23, pp. 39–71. ISBN 978-94-024-1030-3. [Google Scholar]
- Sonego, M.; Madia, M.; Eder, M.; Fleck, C.; Pessan, L.A. Microstructural Features Influencing the Mechanical Performance of the Brazil Nut (Bertholletia excelsa) Mesocarp. J. Mech. Behav. Biomed. Mater. 2021, 116, 104306. [Google Scholar] [CrossRef]
- Francis, D.; Tatam, R.P.; Groves, R.M. Shearography technology and applications: A review. Meas. Sci. Technol. 2010, 21, 102001. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Doube, M.; Kłosowski, M.M.; Arganda-Carreras, I.; Cordelières, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Buttler, B. Finite Element Model to Simulate Elastic Compression Testing on Brazil Nutshell Structures (Bertholletia excelsa). Bachelor’s Thesis, Technische Universität Berlin, Berlin, Germany, 2019. [Google Scholar]
- Green, D.W.; Winandy, J.E.; Kretschmann, D.E. Mechanical Properties of Wood. In Wood Handbook: Wood as an Engineering Material; Forest Products Laboratory, United States Department of Agriculture Forest Service: Madison, WI, USA, 1999; Volume 113, p. 463. [Google Scholar]
- Duffy, S.F.; Baker, E.H.; Wereszczak, A.A.; Swab, J.J. Weibull Analysis Effective Volume and Effective Area for a Ceramic C-Ring Test Specimen. J. Test. Eval. 2005, 33, 12617. [Google Scholar] [CrossRef]
- ASTM C1323-16. Test Method for Ultimate Strength of Advanced Ceramics with Diametrally Compressed C-Ring Specimens at Ambient Temperature. Available online: http://www.astm.org/cgi-bin/resolver.cgi?C1323-16 (accessed on 8 October 2023).
- Matsumoto, N.; Nairn, J.A. Fracture Toughness of Wood and Wood Composites during Crack Propagation. Wood Fiber Sci. 2012, 44, 13. [Google Scholar]
- Ashby, M.F.; Earsterling, F.R.S.; Earsterling, K.E.; Harrysson, R.; Maiti, S.K. The Fracture and Toughness of Woods. Proc. R. Soc. Lond. A 1985, 398, 261–280. [Google Scholar]










| Force | Specimen | E [GPa] | σf [MPa] |
|---|---|---|---|
| Compression | Latitudinal | 2.3 ± 0.3 | 20.1 ± 3.8 |
| Longitudinal | 4.0 ± 1.0 | 26.1 ± 3.9 | |
| Tension | Latitudinal | 2.0 ± 0.3 | 16.2 ± 3.3 |
| Longitudinal | 3.7 ± 0.5 | 26.4 ± 3.6 |
| Longitudinal | Latitudinal | |
|---|---|---|
| Maximum force [kN] | 26.0 ± 2.8 | 15.6 ± 3.3 |
| Displacement at break [mm] | 9.8 ± 3.3 | 4.5 ± 1.0 |
| Specimen | Void Content [%] |
|---|---|
| Non-tested mesocarp | 12.8 ± 2.1 |
| Latitudinal specimen | 13.7 ± 2.7 |
| Longitudinal specimen–peduncle | 12.7 ± 2.4 |
| Longitudinal specimen–opercular opening | 11.5 ± 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonego, M.; Morgenthal, A.; Fleck, C.; Pessan, L.A. Betholletia excelsa Fruit: Unveiling Toughening Mechanisms and Biomimetic Potential for Advanced Materials. Biomimetics 2023, 8, 509. https://doi.org/10.3390/biomimetics8070509
Sonego M, Morgenthal A, Fleck C, Pessan LA. Betholletia excelsa Fruit: Unveiling Toughening Mechanisms and Biomimetic Potential for Advanced Materials. Biomimetics. 2023; 8(7):509. https://doi.org/10.3390/biomimetics8070509
Chicago/Turabian StyleSonego, Marilia, Anneke Morgenthal, Claudia Fleck, and Luiz Antonio Pessan. 2023. "Betholletia excelsa Fruit: Unveiling Toughening Mechanisms and Biomimetic Potential for Advanced Materials" Biomimetics 8, no. 7: 509. https://doi.org/10.3390/biomimetics8070509