Influence of a Physiologically Formed Blood Clot on Pre-Osteoblastic Cells Grown on a BMP-7-Coated Nanoporous Titanium Surface
Abstract
:1. Introduction
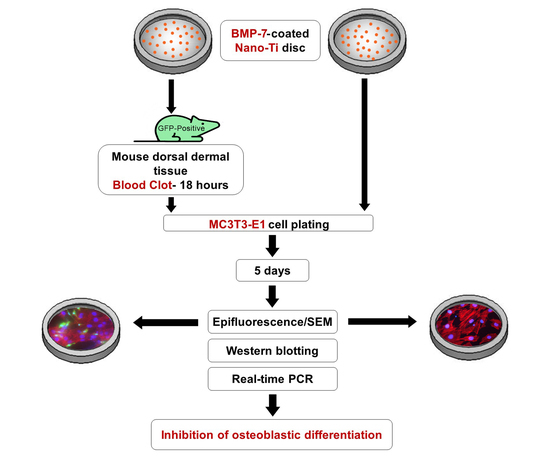
2. Materials and Methods
2.1. Preparation of a Nanotopographic Titanium Surface (Nano-Ti)
2.2. Functionalization of rmBMP-7 on Nano-Ti
2.3. Physiological Blood Clot Formation on Nano-Ti
2.4. Pre-Osteoblastic MC3T3-E1 Cell Culture
2.5. Cell Morphology by Epifluorescence Microscopy
2.6. Cell Morphology by Scanning Electron Microscopy (SEM)
2.7. Quantitative Real-Time Polymerase Chain Reaction (Real-Time PCR)
2.8. Western Blotting (WB)
3. Results
3.1. Epifluorescence and SEM Imaging
3.2. Quantitative mRNA Expression by Real-Time PCR
3.3. Protein Detection by WB
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmes, C.; Tabrizian, M. Surface Functionalization of Biomaterials. In Stem Cell Biology and Tissue Engineering in Dental Sciences; Vishwakarma, A., Sharpe, P., Shi, S., Ramalingam, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 187–206. ISBN 9780123971579. [Google Scholar] [CrossRef]
- Ferraris, S.; Cazzola, M.; Zuardi, L.R.; de Oliveira, P.T. Metal nanoscale systems functionalized with organic compounds. In Nanostructured Biomaterials for Regenerative Medicine; Woodhead Publishing Series in Biomaterials; Guarino, V., Iafisco, M., Spriano, S., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 407–436. ISBN 9780081025949. [Google Scholar] [CrossRef]
- Jablonská, E.; Horkavcová, D.; Rohanová, D.; Brauer, D.S. A review of in vitro cell culture testing methods for bioactive glasses and other biomaterials for hard tissue regeneration. J. Mater. Chem. B 2020, 8, 10941–10953. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M. Future challenges in the in vitro and in vivo evaluation of biomaterial biocompatibility. Regen. Biomater. 2016, 3, 73–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jablonská, E.; Kubásek, J.; Vojtěch, D.; Ruml, T.; Lipov, J. Test conditions can significantly affect the results of in vitro cytotoxicity testing of degradable metallic biomaterials. Sci. Rep. 2021, 11, 6628. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, M.A.; Waser, J.; Milleret, V.; Gerber, I.; Emmert, M.Y.; Foolen, J.; Hoerstrup, S.P.; Schlottig, F.; Vogel, V. Synergistic interactions of blood-borne immune cells, fibroblasts and extracellular matrix drive repair in an in vitro peri-implant wound healing model. Sci. Rep. 2016, 6, 21071. [Google Scholar] [CrossRef] [Green Version]
- Lackington, W.A.; Fleyshman, L.; Schweizer, P.; Elbs-Glatz, Y.; Guimond, S.; Rottmar, M. The response of soft tissue cells to Ti implants is modulated by blood-implant interactions. Mater. Today Bio. 2022, 15, 100303. [Google Scholar] [CrossRef] [PubMed]
- Al-Jarsha, M.; Moulisová, V.; Leal-Egaña, A.; Connell, A.; Naudi, K.B.; Ayoub, A.F.; Dalby, M.J.; Salmerón-Sánchez, M. Engineered coatings for titanium implants to present ultralow doses of BMP-7. ACS Biomater. Sci. Eng. 2018, 4, 1812–1819. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, J.; Wu, D.; Yu, J. Surface functionalization of titanium with Bmp-7/rgd/hyaluronic acid for promoting osteoblast functions. J. Biomater. Tissue Eng. 2019, 9, 32–39. [Google Scholar] [CrossRef]
- Nemcakova, I.; Litvinec, A.; Mandys, V.; Potocky, S.; Plencner, M.; Doubkova, M.; Nanka, O.; Olejnickova, V.; Sankova, B.; Bartos, M.; et al. Coating Ti6Al4V implants with nanocrystalline diamond functionalized with BMP-7 promotes extracellular matrix mineralization in vitro and faster osseointegration in vivo. Sci. Rep. 2022, 12, 5264. [Google Scholar] [CrossRef]
- López-Valverde, N.; Aragoneses, J.; López-Valverde, A.; Rodríguez, C.; Aragoneses, J.M. Role of BMP-7 on biological parameters osseointegration of dental implants: Preliminary results of a preclinical study. Front. Bioeng. Biotechnol. 2023, 11, 1153631. [Google Scholar] [CrossRef]
- Zuardi, L.R.; de Oliveira, F.S.; Fernandes, R.R.; Gomes, M.P.O.; Spriano, S.; Nanci, A.; de Oliveira, P.T. Effects of rmBMP-7 on osteoblastic cells grown on a nanostructured titanium surface. Biomimetics 2022, 7, 136. [Google Scholar] [CrossRef]
- Milleret, V.; Tugulu, S.; Schlottig, F.; Hall, H. Alkali treatment of microrough titanium surfaces affects macrophage/monocyte adhesion, platelet activation and architecture of blood clot formation. Eur. Cells Mater. 2011, 21, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Chen, J.Y.; Lin, M.C.; Wang, Y.T.; Lee, T.L.; Chen, L.K. Blood responses to titanium surface with TiO2 nano-mesh structure. Clin. Oral Implant. Res. 2012, 23, 379–383. [Google Scholar] [CrossRef]
- Anitua, E.; Prado, R.; Orive, G.; Tejero, R. Effects of calcium-modified titanium implant surfaces on platelet activation, clot formation, and osseointegration. J. Biomed. Mater. Res. A 2015, 103, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Kopf, B.S.; Schipanski, A.; Rottmar, M.; Berner, S.; Maniura-Weber, K. Enhanced differentiation of human osteoblasts on Ti surfaces pre-treated with human whole blood. Acta Biomater. 2015, 19, 180–190. [Google Scholar] [CrossRef] [PubMed]
- VanZweden, E.; Tolsma, R.; Hung, V.; Awad, P.; Sawyer, R.; Li, Y. The advances of blood clots used as biomaterials in regenerative medicine. Regen. Med. 2022, 17, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Monroe, D.M.; Hoffman, M. The clotting system—A major player in wound healing. Haemophilia 2012, 18 (Suppl. 5), 11–16. [Google Scholar] [CrossRef]
- Vetrone, F.; Variola, F.; de Oliveira, P.T.; Zalzal, S.F.; Yi, J.H.; Sam, J.; Bombonato-Prado, K.F.; Sarkissian, A.; Perepichka, D.F.; Wuest, J.D.; et al. Nanoscale oxidative patterning of metallic surfaces to modulate cell activity and fate. Nano Lett. 2009, 9, 659–665. [Google Scholar] [CrossRef]
- Scannavino, R.C.P.; Riccucci, G.; Ferraris, S.; Duarte, G.L.C.; de Oliveira, P.T.; Spriano, S. Functionalization with polyphenols of a nano-textured Ti surface through a high-amino acid medium: A chemical-physical and biological characterization. Nanomaterials 2022, 12, 2916. [Google Scholar] [CrossRef]
- de Oliveira, P.T.; Zalzal, S.F.; Beloti, M.M.; Rosa, A.L.; Nanci, A. Enhancement of in vitro osteogenesis on titanium by chemcally produced nanotopography. J. Biomed. Mater. Res. A 2007, 80, 554–564. [Google Scholar] [CrossRef]
- Guadarrama Bello, D.; Fouillen, A.; Badia, A.; Nanci, A. A nanoporous titanium surface promotes the maturation of focal adhesions and formation of filopodia with distinctive nanoscale protrusions by osteogenic cells. Acta Biomater. 2017, 60, 339–349. [Google Scholar] [CrossRef]
- Guadarrama Bello, D.; Fouillen, A.; Badia, A.; Nanci, A. Nanoporosity Stimulates Cell Spreading and Focal Adhesion Formation in Cells with Mutated Paxillin. ACS Appl. Mater. Interfaces 2020, 12, 14924–14932. [Google Scholar] [CrossRef]
- Bueno, R.B.; Teixeira, L.N.; De Almeida, A.L.; Soares, A.C.; Beloti, M.M.; Sverzut, C.E.; De Oliveira, O.N.; Nanci, A.; Rosa, A.L.; de Oliveira, P.T. Growth and differentiation factor 5 (GDF-5)-functionalized, nanostructured titanium surfaces: In vitro and in vivo studies. In Proceedings of the 10th World Biomaterials Congress, Montréal, QC, Canada, 17–22 May 2016. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Maliakal, J.C.; Asahina, I.; Hauschka, P.V.; Sampath, T.K. Osteogenic protein-1 (BMP-7) inhibits cell proliferation and stimulates the expression of markers characteristic of osteoblast phenotype in rat osteosarcoma (17/2.8) cells. Growth Factors 1994, 11, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ren, L.F.; Lin, H.S.; Yin, M.N.; Tong, Y.Q.; Shi, G.S. The optimal dose of recombinant human osteogenic protein-1 enhances differentiation of mouse osteoblast-like cells: An in vitro study. Arch. Oral Biol. 2012, 57, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, A.; Klein, A.; Ritz, U.; Ackermann, A.; Anthonissen, J.; Kaufmann, K.B.; Brendel, C.; Götz, H.; Rommens, P.M.; Hofmann, A. Surface Functionalization of Orthopedic Titanium Implants with Bone Sialoprotein. PLoS ONE 2016, 11, e0153978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopf, B.S.; Ruch, S.; Berner, S.; Spencer, N.D.; Maniura-Weber, K. The role of nanostructures and hydrophilicity in osseointegration: In-vitro protein-adsorption and blood-interaction studies. J. Biomed. Mater. Res. A 2015, 103, 2661–2672. [Google Scholar] [CrossRef]
- Di Iorio, D.; Traini, T.; Degidi, M.; Caputi, S.; Neugebauer, J.; Piattelli, A. Quantitative evaluation of the fibrin clot extension on different implant surfaces: An in vitro study. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 636–642. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef]
- Bujoli, B.; Scimeca, J.C.; Verron, E. Fibrin as a Multipurpose Physiological Platform for Bone Tissue Engineering and Targeted Delivery of Bioactive Compounds. Pharmaceutics 2019, 11, 556. [Google Scholar] [CrossRef] [Green Version]
- Pagel, C.N.; Sivagurunathan, S.; Loh, L.H.; Tudor, E.M.; Pike, R.N.; Mackie, E.J. Functional responses of bone cells to thrombin. Biol. Chem. 2006, 387, 1037–1041. [Google Scholar] [CrossRef]
- Altman, R.; Scazziota, A.S.; Herrera, M.D.L.; Gonzalez, C. Thrombin generation by activated factor VII on platelet activated by different agonists. Extending the cell-based model of hemostasis. Thromb. J. 2006, 4, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, S.; Hayashi, M.; Komiya, S.; Imamura, T.; Miyazono, K. Endogenous TGF-beta signaling suppresses maturation of osteoblastic mesenchymal cells. EMBO J. 2004, 23, 552–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, P.T.; de Oliva, M.A.; Maximiano, W.M.; Sebastião, K.E.; Crippa, G.E.; Ciancaglini, P.; Beloti, M.M.; Nanci, A.; Rosa, A.L. Effects of a mixture of growth factors and proteins on the development of the osteogenic phenotype in human alveolar bone cell cultures. J. Histochem. Cytochem. 2008, 56, 629–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey Dubose, K.; Zayzafoon, M.; Murphy-Ullrich, J.E. Thrombospondin-1 inhibits osteogenic differentiation of human mesenchymal stem cells through latent TGF-β activation. Biochem. Biophys. Res. Commun. 2012, 422, 488–493. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhang, Y.; Yin, Q.; Xia, H.; Wang, J. Platelet-derived growth factor promotes osteoblast proliferation by activating G-protein-coupled receptor kinase interactor-1. Mol. Med. Rep. 2014, 10, 1349–1354. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, J.; Horowitz, M.; Choi, Y. Osteoimmunology: Interactions of the bone and immune system. Endocr. Rev. 2008, 29, 403–440. [Google Scholar] [CrossRef] [Green Version]
- Yang, N.; Liu, Y. The Role of the Immune Microenvironment in Bone Regeneration. Int. J. Med. Sci. 2021, 18, 3697–3707. [Google Scholar] [CrossRef]
- Huang, W.; Carlsen, B.; Rudkin, G.; Berry, M.; Ishida, K.; Yamaguchi, D.T.; Miller, T.A. Osteopontin is a negative regulator of proliferation and differentiation in MC3T3-E1 pre-osteoblastic cells. Bone 2004, 34, 799–808. [Google Scholar] [CrossRef]
- Senger, D.R.; Perruzzi, C.A.; Papadopoulos-Sergiou, A.; Van de Water, L. Adhesive properties of osteopontin: Regulation by a naturally occurring thrombin-cleavage in close proximity to the GRGDS cell-binding domain. Mol. Biol. Cell 1994, 5, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Agnihotri, R.; Crawford, H.C.; Haro, H.; Matrisian, L.M.; Havrda, M.C.; Liaw, L. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin). J. Biol. Chem. 2001, 276, 28261–28267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, B.; Schack, L.; Kläning, E.; Sørensen, E.S. Osteopontin is cleaved at multiple sites close to its integrin-binding motifs in milk and is a novel substrate for plasmin and cathepsin D. J. Biol. Chem. 2010, 285, 7929–7937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licari, L.G.; Kovacic, J.P. Thrombin physiology and pathophysiology. J. Vet. Emerg. Crit. Care 2009, 19, 11–22. [Google Scholar] [CrossRef]
- Kremers, R.M.; Wagenvoord, R.J.; Hemker, H.C. The effect of fibrin(ogen) on thrombin generation and decay. Thromb. Haemost. 2014, 112, 486–494. [Google Scholar] [CrossRef]
- Guo, J.; Lin, Q.; Shao, Y.; Rong, L.; Zhang, D. BMP-7 suppresses excessive scar formation by activating the BMP-7/Smad1/5/8 signaling pathway. Mol. Med. Rep. 2017, 16, 1957–1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruendler, C.; Lin, Y.; Farley, J.; Wang, T. Proteasomal degradation of Smad1 induced by bone morphogenetic proteins. J. Biol. Chem. 2001, 276, 46533–46543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Kavsak, P.; Abdollah, S.; Wrana, J.L.; Thomsen, G.H. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature 1999, 400, 687–693. [Google Scholar] [CrossRef]
- Dong, X.; Wang, Q.; Wu, T.; Pan, H. Understanding adsorption-desorption dynamics of BMP-2 on hydroxyapatite (001) surface. Biophys. J. 2007, 93, 750–759. [Google Scholar] [CrossRef] [Green Version]
- Wagner, I.; Wang, H.; Weissert, P.M.; Straube, W.L.; Shevchenko, A.; Gentzel, M.; Brito, G.; Tazaki, A.; Oliveira, C.; Sugiura, T.; et al. Serum proteases potentiate BMP-induced cell cycle re-entry of dedifferentiating muscle cells during newt limb regeneration. Dev. Cell 2017, 40, 608–617.e6. [Google Scholar] [CrossRef] [Green Version]




| Groups |
Animals per Group |
Animals per Analysis |
Discs per Analysis |
|---|---|---|---|
| MC3T3-E1 | - | - | WB (20) PCR (16) Morphology (4) |
| MC3T3-E1 + BMP-7 | - | - | |
| Clot * | 20 | WB (10) PCR (8) Morphology (2) | |
| Clot + MC3T3-E1 | 20 | ||
| Clot + MC3T3-E1 + BMP-7 | 20 | ||
| TOTAL | 60 | 60 | 200 |
| Genes | Taqman Probes |
|---|---|
| Runx2 | Mm00501584_m1 |
| Osx | Mm04933803_m1 |
| Alp | Mm00475834_m1 |
| Bsp | Mm00492555_m1 |
| Oc | Mm03413826_mH |
| Opn | Mm00436767_m1 |
| Gapdh | Mm99999915_g1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuardi, L.R.; Silva, C.L.A.; Rego, E.M.; Carneiro, G.V.; Spriano, S.; Nanci, A.; de Oliveira, P.T. Influence of a Physiologically Formed Blood Clot on Pre-Osteoblastic Cells Grown on a BMP-7-Coated Nanoporous Titanium Surface. Biomimetics 2023, 8, 123. https://doi.org/10.3390/biomimetics8010123
Zuardi LR, Silva CLA, Rego EM, Carneiro GV, Spriano S, Nanci A, de Oliveira PT. Influence of a Physiologically Formed Blood Clot on Pre-Osteoblastic Cells Grown on a BMP-7-Coated Nanoporous Titanium Surface. Biomimetics. 2023; 8(1):123. https://doi.org/10.3390/biomimetics8010123
Chicago/Turabian StyleZuardi, Leonardo Raphael, Cleide Lúcia Araújo Silva, Eduardo Magalhães Rego, Giovana Vacilotto Carneiro, Silvia Spriano, Antonio Nanci, and Paulo Tambasco de Oliveira. 2023. "Influence of a Physiologically Formed Blood Clot on Pre-Osteoblastic Cells Grown on a BMP-7-Coated Nanoporous Titanium Surface" Biomimetics 8, no. 1: 123. https://doi.org/10.3390/biomimetics8010123