Green Synthesis of Hierarchically Porous Carbon Derived from Coal Tar Pitch for Enhanced Lithium Storage
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
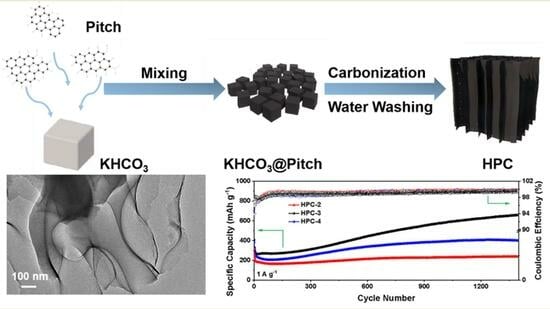
2.2. Synthesis of HPC
2.3. Material Characterizations
2.4. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Larcher, D.; Tarascon, J.M. Towards Greener and More Sustainable Batteries for Electrical Energy Storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Gur, T.M. Review of Electrical Energy Storage Technologies, Materials and Systems: Challenges and Prospects for Large-Scale Grid Storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and Fuel Cells for Emerging Electric Vehicle Markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Li, L.; Zhang, D.; Deng, J.; Gou, Y.; Fang, J.; Cui, H.; Zhao, Y.; Cao, M. Carbon-Based Materials for Fast Charging Lithium-Ion Batteries. Carbon 2021, 183, 721–734. [Google Scholar] [CrossRef]
- Xie, L.; Tang, C.; Bi, Z.; Song, M.; Fan, Y.; Yan, C.; Li, X.; Su, F.; Zhang, Q.; Chen, C. Hard Carbon Anodes for Next-Generation Li-Ion Batteries: Review and Perspective. Adv. Energy Mater. 2021, 11, 2101650. [Google Scholar] [CrossRef]
- Long, W.; Fang, B.; Ignaszak, A.; Wu, Z.; Wang, Y.-J.; Wilkinson, D. Biomass-Derived Nanostructured Carbons and Their Composites as Anode Materials for Lithium Ion Batteries. Chem. Soc. Rev. 2017, 46, 7176–7190. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The Success Story of Graphite as a Lithium-Ion Anode Material-Fundamentals, Remaining Challenges, and Recent Developments Including Silicon (Oxide) Composites. Sustain. Energ. Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Hassoun, J.; Bonaccorso, F.; Agostini, M.; Angelucci, M.; Betti, M.G.; Cingolani, R.; Gemmi, M.; Mariani, C.; Panero, S.; Pellegrini, V.; et al. An Advanced Lithium-Ion Battery Based on a Graphene Anode and a Lithium Iron Phosphate Cathode. Nano Lett. 2014, 14, 4901–4906. [Google Scholar] [CrossRef]
- Xing, Z.; Ju, Z.; Zhao, Y.; Wan, J.; Zhu, Y.; Qiang, Y.; Qian, Y. One-Pot Hydrothermal Synthesis of Nitrogen-Doped Graphene as High-Performance Anode Materials for Lithium Ion Batteries. Sci. Rep. 2016, 6, 26146. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Tan, W.K.; Kar, K.K.; Matsuda, A. Recent Progress in the Synthesis of Graphene and Derived Materials for Next Generation Electrodes of High Performance Lithium Ion Batteries. Prog. Energy. Combust. Sci. 2019, 75, 100786. [Google Scholar] [CrossRef]
- Jessl, S.; Copic, D.; Engelke, S.; Ahmad, S.; De Volder, M. Hydrothermal Coating of Patterned Carbon Nanotube Forest for Structured Lithium-Ion Battery Electrodes. Small 2019, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, M.; Feng, Z.; Huang, Y.; Qian, T.; Hu, H.; Zheng, X.; Liu, P.; Liu, H.; Xing, T.; et al. Anchoring Perovskite-Type FeMnO3 Microspheres on CNT Conductive Networks via Electrostatic Self-Assembly for High-Performance Lithium-Ion Capacitors. Chem. Eng. J. 2021, 424, 130315. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, K.; Zhu, K.; Liu, X.; Ye, K.; Yan, J.; Wang, G.; Cao, D. Cable-Like Polyimide@Carbon Nanotubes Composite as a Capable Anode for Lithium Ion Batteries. Chem. Eng. J. 2022, 446, 137208. [Google Scholar] [CrossRef]
- Pramanik, A.; Chattopadhyay, S.; De, G.; Mahanty, S. Design of Cuboidal FeNi2S4-RGO-MWCNTs Composite for Lithium-Ion Battery Anode Showing Excellent Half and Full Cell Performances. Batteries 2022, 8, 261. [Google Scholar] [CrossRef]
- Zhao, Z.; Das, S.; Xing, G.; Fayon, P.; Heasman, P.; Jay, M.; Bailey, S.; Lambert, C.; Yamada, H.; Wakihara, T.; et al. A 3D Organically Synthesized Porous Carbon Material for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2018, 57, 11952–11956. [Google Scholar] [CrossRef]
- Qiu, D.; Kang, C.; Li, M.; Wei, J.; Hou, Z.; Wang, F.; Yang, R. Biomass-Derived Mesopore-Dominant Hierarchical Porous Carbon Enabling Ultra-Efficient Lithium Ion Storage. Carbon 2020, 162, 595–603. [Google Scholar] [CrossRef]
- Mondal, A.K.; Kretschmer, K.; Zhao, Y.; Liu, H.; Wang, C.; Sun, B.; Wang, G. Nitrogen-Doped Porous Carbon Nanosheets from Eco-Friendly Eucalyptus Leaves as High Performance Electrode Materials for Supercapacitors and Lithium Ion Batteries. Chem. Eur. J. 2017, 23, 3683–3690. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Y.; Chen, C.; Wang, M.; Liu, P. Phosphorus-Doped Porous Biomass Carbon with Ultra-Stable Performance in Sodium Storage and Lithium Storage. Electrochim. Acta 2019, 321, 134698. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, Z.; Yu, H.; Zhang, X.; Liu, T.; Xia, M.; Zheng, R.; Shui, M.; Shu, J. Heteroatom-Doped Carbon-Based Materials for Lithium and Sodium Ion Batteries. Energy Storage Mater. 2020, 32, 65–90. [Google Scholar] [CrossRef]
- Fu, F.; He, Q.; Zhang, X.; Key, J.; Shen, P.; Zhu, J. Facile Synthesis of Nickel Phosphide@N-Doped Carbon Nanorods with Exceptional Cycling Stability as Li-Ion and Na-Ion Battery Anode Material. Batteries 2023, 9, 267. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, T. Recent Advances in Inorganic 2D Materials and Their Applications in Lithium and Sodium Batteries. J. Mater. Chem. A 2017, 5, 3735–3758. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, C.; Yang, J.; Zhao, C.; Ling, Z.; Qiu, J. Nitrogen-Doped Tubular/Porous Carbon Channels Implanted on Graphene Frameworks for Multiple Confinement of Sulfur and Polysulfides. J. Mater. Chem. A 2017, 5, 10380–10386. [Google Scholar] [CrossRef]
- He, Y.; Zhuang, X.; Lei, C.; Lei, L.; Hou, Y.; Mai, Y.; Feng, X. Porous Carbon Nanosheets: Synthetic Strategies and Electrochemical Energy Related Applications. Nano Today 2019, 24, 103–119. [Google Scholar] [CrossRef]
- Jiang, M.; Sun, N.; Ali Soomro, R.; Xu, B. The Recent Progress of Pitch-Based Carbon Anodes in Sodium-Ion Batteries. J. Energy Chem. 2021, 55, 34–47. [Google Scholar] [CrossRef]
- Yuan, M.; Cao, B.; Meng, C.; Zuo, H.; Li, A.; Ma, Z.; Chen, X.; Song, H. Preparation of Pitch-Based Carbon Microbeads by a Simultaneous Spheroidization and Stabilization Process for Lithium-Ion Batteries. Chem. Eng. J. 2020, 400, 125948. [Google Scholar] [CrossRef]
- Zhang, G.; Guan, T.; Qiao, J.; Wang, J.; Li, K. Free-Radical-Initiated Strategy Aiming for Pitch-Based Dual-Doped Carbon Nanosheets Engaged into High-Energy Asymmetric Supercapacitors. Energy Storage Mater. 2020, 26, 119–128. [Google Scholar] [CrossRef]
- Geng, W.; Ma, F.; Wu, G.; Song, S.; Wan, J.; Ma, D. MgO-Templated Hierarchical Porous Carbon Sheets Derived from Coal Tar Pitch for Supercapacitors. Electrochim. Acta 2016, 191, 854–863. [Google Scholar] [CrossRef]
- He, X.; Li, X.; Ma, H.; Han, J.; Zhang, H.; Yu, C.; Xiao, N.; Qiu, J. ZnO Template Strategy for the Synthesis of 3D Interconnected Graphene Nanocapsules from Coal Tar Pitch as Supercapacitor Electrode Materials. J. Power Sources 2017, 340, 183–191. [Google Scholar] [CrossRef]
- Xing, B.; Zhang, C.; Liu, Q.; Zhang, C.; Huang, G.; Guo, H.; Cao, J.; Cao, Y.; Yu, J.; Chen, Z. Green Synthesis of Porous Graphitic Carbons from Coal Tar Pitch Templated by Nano-CaCO3 for High-Performance Lithium-Ion Batteries. J. Alloys Compd. 2019, 795, 91–102. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, H.; Li, Y.; Qi, D.; Wang, S.; Shen, K. Hierarchical Porous Carbon Derived from Carboxylated Coal-Tar Pitch for Electrical Double-Layer Capacitors. RSC Adv. 2019, 9, 29131–29140. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, S.; Zhang, Z.; Chi, Y.; Yang, C.; Wang, C.; Zhen, Y.; Wang, D.; Fu, F.; Chi, R.A. Pore Structure Regulation of Hierarchical Porous Carbon Derived from Coal Tar Pitch Via Pre-Oxidation Strategy for High-Performance Supercapacitor. J. Colloid Interf. Sci. 2022, 614, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zuo, P.; Qu, S. Adjusting Hydrophily and Aromaticity Strategy for Pitch-Based Hierarchical Porous Carbon and Its Application in Flexible Supercapacitor. Fuel 2022, 311, 122514. [Google Scholar] [CrossRef]
- Qian, T.; Huang, Y.; Zhang, M.; Xia, Z.; Liu, H.; Guan, L.; Hu, H.; Wu, M. Non-Corrosive and Low-Cost Synthesis of Hierarchically Porous Carbon Frameworks for High-Performance Lithium-Ion Capacitors. Carbon 2021, 173, 646–654. [Google Scholar] [CrossRef]
- Huang, S.; Li, Z.; Wang, B.; Zhang, J.; Peng, Z.; Qi, R.; Wang, J.; Zhao, Y. N-Doping and Defective Nanographitic Domain Coupled Hard Carbon Nanoshells for High Performance Lithium/Sodium Storage. Adv. Funct. Mater. 2018, 28, 1706294. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, X.; Tian, X.; Liu, Z. Coal-Based Semicoke-Derived Carbon Anode Materials with Tunable Microcrystalline Structure for Fast Lithium-Ion Storage. Nanomaterials 2022, 12, 4067. [Google Scholar] [CrossRef]
- Mu, J.; Zhang, M.; Li, Y.; Dong, Z.; Pan, Y.; Chen, B.; He, Z.H.; Fang, H.; Kong, S.; Gu, X.; et al. Laser Irradiation Constructing All-in-One Defective Graphene-Polyimide Separator for Effective Restraint of Lithium Dendrites and Shuttle Effect. Nano Res. 2023. [Google Scholar] [CrossRef]
- Shi, Z.; Sun, G.; Yuan, R.; Chen, W.; Wang, Z.; Zhang, L.; Zhan, K.; Zhu, M.; Yang, J.; Zhao, B. Scalable Fabrication of NiCO2O4/Reduced Graphene Oxide Composites by Ultrasonic Spray as Binder-Free Electrodes for Supercapacitors with Ultralong Lifetime. J. Mater. Sci. Technol. 2022, 99, 260–269. [Google Scholar] [CrossRef]
- Peng, H.; Yao, B.; Wei, X.; Liu, T.; Kou, T.; Xiao, P.; Zhang, Y.; Li, Y. Pore and Heteroatom Engineered Carbon Foams for Supercapacitors. Adv. Energy Mater. 2019, 9, 1803665. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Y.; Zhu, Y.; Zhang, Z.; Guang, Z.; Meng, Z.; Liu, P. Nonignorable Influence of Oxygen in Hard Carbon for Sodium Ion Storage. Acs Sustain. Chem. Eng. 2020, 8, 1497–1506. [Google Scholar] [CrossRef]
- Gao, F.; Geng, C.; Xiao, N.; Qu, J.; Qiu, J. Hierarchical Porous Carbon Sheets Derived from Biomass Containing an Activation Agent and in-Built Template for Lithium Ion Batteries. Carbon 2018, 139, 1085–1092. [Google Scholar] [CrossRef]
- Huang, Y.; Fang, Y.; Lu, X.F.; Luan, D.; Lou, X.W. Co3O4 Hollow Nanoparticles Embedded in Mesoporous Walls of Carbon Nanoboxes for Efficient Lithium Storage. Angew. Chem. Int. Ed. 2020, 59, 19914–19918. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Duan, H.; Xu, B.; Zhang, L.; Hu, Y.; Zhao, C.; Wang, Z.; Chen, L.; Yang, Y. Lithium Storage in Nitrogen-Rich Mesoporous Carbon Materials. Energy Environ. Sci. 2012, 5, 7950–7955. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, X.; Mu, J.; Liu, P.; Yuan, W.; Li, S.; Wang, X.; Fang, H.; Liu, H.; Xing, T.; et al. Robust and Fast Lithium Storage Enabled by Polypyrrole-Coated Nitrogen and Phosphorus Co-Doped Hollow Carbon Nanospheres for Lithium-Ion Capacitors. Front. Chem. 2021, 9, 760473. [Google Scholar] [CrossRef]
- Zhang, M.; Mu, J.; Li, Y.; Pan, Y.; Dong, Z.; Chen, B.; Guo, S.; Yuan, W.; Fang, H.; Hu, H.; et al. Propelling Polysulfide Redox by Fe3C-FeN Heterostructure@Nitrogen-Doped Carbon Framework Towards High-Efficiency Li-S Batteries. J. Energy Chem. 2023, 78, 105–114. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Z.; Xing, B.; Zeng, H.; Gao, R.; Huang, G.; Jia, J.; Cao, Y.; Zhang, C. Carbon Nanosheets Prepared with a Vermiculite Template for High-Performance Lithium-Ion Batteries Via Space-Confined Carbonization Strategy. J. Alloys Compd. 2023, 933, 167721. [Google Scholar] [CrossRef]
- Han, L.; Zhu, X.; Yang, F.; Liu, Q.; Jia, X. Eco-Conversion of Coal into a Nonporous Graphite for High-Performance Anodes of Lithium-Ion Batteries. Powder Technol. 2021, 382, 40–47. [Google Scholar] [CrossRef]
- Xie, M.; Zhu, X.; Li, D.; Xu, Z.; Huang, Y.; Zha, H.; Ding, M.; Jia, C. Spent Asphalt-Derived Mesoporous Carbon for High-Performance Li/Na/K-Ion Storage. J. Power Sources 2021, 514, 230593. [Google Scholar] [CrossRef]
- Alvin, S.; Cahyadi, H.S.; Hwang, J.; Chang, W.; Kwak, S.K.; Kim, J. Revealing the Intercalation Mechanisms of Lithium, Sodium, and Potassium in Hard Carbon. Adv. Energy Mater. 2020, 10, 2000283. [Google Scholar] [CrossRef]
- Tian, H.; Zhao, L.; Wang, L.; Xia, Z.; Tan, W.; Jiao, Z. Bimetallic Flower-Like NiCoP Encapsulated in an N-Doped Carbon Shell with Enhanced Lithium Storage Properties. Batteries 2023, 9, 361. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Xu, C.; Jiang, H.; Li, C.; Zhang, L.; Lin, J.; Shen, Z.X. Advanced Energy Storage Devices: Basic Principles, Analytical Methods, and Rational Materials Design. Adv. Sci. 2018, 5, 1700322. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Kang, X.; Wang, F.; Zhang, J.; Zhang, T.; Ran, F. Capacitive Contribution Matters in Facilitating High Power Battery Materials toward Fast-Charging Alkali Metal Ion Batteries. Mater. Sci. Eng. R-Rep. 2023, 154, 100737. [Google Scholar] [CrossRef]





| Samples | d002 (nm) | ID/IG | SBET (a) (m2 g−1) | Smic (b) (m2 g−1) | Vt (c) (cm3 g−1) | Vmic (d) (cm3 g−1) | Vmic/Vt (e) (%) |
|---|---|---|---|---|---|---|---|
| HPC-2 | 0.378 | 0.91 | 1048 | 877 | 0.56 | 0.43 | 76.8 |
| HPC-3 | 0.382 | 0.97 | 1643 | 751 | 0.99 | 0.36 | 36.4 |
| HPC-4 | 0.380 | 0.94 | 986 | 776 | 0.64 | 0.38 | 59.4 |
| Anode Material | Precursor | Method | Cycling Performance | Ref. |
|---|---|---|---|---|
| HPC-3 | CTP | KHCO3 template | 1 A g−1, 660 mAh g−1 after 1400 cycles | This work |
| N-doped porous carbon nanosheets | Eucalyptus leaves | KHCO3 template | 0.5 A g−1, 513 mAh g−1 after 500 cycles | [18] |
| Porous graphitic carbons | CTP | CaCO3 template | 1 A g−1, 391 mAh g−1 after 1000 cycles | [30] |
| Amorphous carbon | Semicoke | Direct pyrolysis | 1 A g−1, 210 mAh g−1 after 1000 cycles | [36] |
| Hierarchical porous carbon sheets | Soft pitch | Oyster shell template | 1 A g−1, 451 mAh g−1 after 450 cycles | [41] |
| Carbon nanosheets | Soluble starch | Vermiculite template | 0.1 A g−1, 522 mAh g−1 after 100 cycles | [46] |
| Nonporous graphite | Anthracite | MgO template /KOH activation | 0.5 A g−1, 174 mAh g−1 after 1000 cycles | [47] |
| Mesoporous carbon | Spent asphalt | Fe2O3 template | 0.2 A g−1, 565 mAh g−1 after 80 cycles | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Qu, M.; Yuan, W.; Mu, J.; He, Z.; Wu, M. Green Synthesis of Hierarchically Porous Carbon Derived from Coal Tar Pitch for Enhanced Lithium Storage. Batteries 2023, 9, 473. https://doi.org/10.3390/batteries9090473
Zhang M, Qu M, Yuan W, Mu J, He Z, Wu M. Green Synthesis of Hierarchically Porous Carbon Derived from Coal Tar Pitch for Enhanced Lithium Storage. Batteries. 2023; 9(9):473. https://doi.org/10.3390/batteries9090473
Chicago/Turabian StyleZhang, Mengdi, Meng Qu, Wenhan Yuan, Jiawei Mu, Zhengqiu He, and Mingbo Wu. 2023. "Green Synthesis of Hierarchically Porous Carbon Derived from Coal Tar Pitch for Enhanced Lithium Storage" Batteries 9, no. 9: 473. https://doi.org/10.3390/batteries9090473